Introduction
Rose is a woody perennial plant, which belongs to genus Rosa and family Rosaceae.1 Rose is number one cut flower in world floriculture trade both in quantity and quality.2 Rose is popular for its beauty, fragrance and long lasting blooming qualities.3 They are used for variety of purposes namely pot plant, garden plant and cut flower production.4
Seaweed is a marine macro-algae. Brown seaweed are the second most abundant group comprising about 2,000 species which are most commonly used in agriculture.5 Ascophyllum spp., Fucus spp., Laminaria spp., Sargassum spp., and Turbinaria spp. are the seaweeds which are used as biofertilizers to improve the plant growth.6 Coastline area of Sri Lanka is consisted of many varieties of seaweeds.
There are many benefits of application of seaweed extract in agriculture. Several studies reported that, the seaweed extract (Sargassum crassifolium L.) has the potential to induce growth and flowering in crops. Srikrishna and Sutharsan7 found that, foliar application of 20% of seaweed extract of Sargassum crassifolium L. increased growth and flowering in Anthurium. Sutharsan et al.,8 reported that, foliar application of 20% seaweed extract (Sargassum crassifolium) increased number of flowers in tomato.
Performance of flowering in roses is very low due to unfavorable climatic and soil conditions. Therefore, it is necessary to induce flowering with the enhancement of vegetative growth in roses to maximize flower production. Hence seaweed liquid extract could be used as a bio stimulant to enhance flowering in roses. It is cost effective and eco-friendly technique. However, optimum concentration and frequency of application of liquid seaweed extract of Sargassum crassifolium L.to promote growth and flowering in roses need to be more investigated. Hence the present study was conducted to select the optimum concentration and frequency of application of seaweed extract (Sargassum crassifolium L.) to enhance the growth and flowering of roses.
Materials and Methods
Collection of Seaweeds (Sargassum crassifolium)
The seaweeds were collected from Pasikudah, coastal area, East of Sri Lanka about 35 km from Batticaloa. Seaweeds were collected by using hand and were immediately washed with sea water to remove the foreign particles, sand particles and epiphytes. It was kept in polythene bag with seawater and immediately transported to Crop Science Laboratory, Eastern University, Sri Lanka. The seaweeds were washed by tap water and then distilled water to remove the salt on the surface. Finally, they were spread on the blotting paper to remove excess water.
Preparation of Seaweed Liquid Extract (SLE)
Shade drying of seaweeds was done for 3-4 days. After shade drying seaweeds were hand crushed to make into small pieces. Then, they were ground to make as coarse powder by using the laboratory blender. Then the material was taken to prepare the seaweed liquid extract (SLE) in the laboratory as per the methodology described by Rama Rao.9 Seaweed powder was added with distilled water in the ratio of 1:20 (W/V) and was autoclaved at 15 lbs/sq and 121oC for a period of 20 minutes. The hot extract was filtered by double-layered cheese cloth and it was allowed to cool at 4 oC. Thereafter, the filtrate was centrifuged (5000 rpm) for 15 minutes. The supernatant was separately collected and it was considered as 100% of seaweed liquid extract. 10%, 20% and 30% concentration of seaweed liquid extract was prepared by adding distilled water at volume basis as per the treatment structure.
Experimental Site
The pot experiment was conducted from June to September 2018 at the Crop Farm, Eastern University, Vantharumoolai, Sri Lanka. The experimental site belongs to low country dry zone (DL2) agro ecological zone. The site is in between the latitude of 7.73ºN and longitude of 81.67ºE.
Experimental Design
The experimental design was Completely Randomized Design (CRD) with seven treatments. Each treatment was replicated ten times. An experimental unit consisted of one plant. The treatments were defined as, once a week application of 10% seaweed liquid extract (T1), twice a week application of 10% seaweed liquid extract (T2), once a week application of 20% seaweed liquid extract (T3), twice a week application of 20% seaweed liquid extract (T4), once a week application of 30% seaweed liquid extract (T5), twice a week application of 30% seaweed liquid extract (T6) and application of distilled water (T7- control). Other management practices were followed uniformly as per the recommendations of Department of National Botanic Gardens, Sri Lanka.
Planting materials and application of seaweed liquid extract
Budded rose plants (var. local) of one year old were obtained from a private nursery. All the plants were pruned to uniform height (35cm) before commencement of the experiment. Seaweeed liquid extract was applied as foliar spray as per treatment structure.
Measurements
Plant height (distance from soil surface in the pot to top most leaf by meter scale), leaf area (leaf area meter, LI-3100C), plant biomass (oven dry method) and number of flowers were measured at monthly interval. Destructive sampling method was practiced.
Statistical Analysis
Analysis of variance (ANOVA) test was used to determine the effects of treatments on measured parameters. Duncan’s multiple range test at 5% probability was used to compare treatments means.
Results
Plant Height
It was found that there were significant (p<0.05) differences in the plant height of rose plants grown under different treatments at 2 and 3 months after transplanting (MAT). Highest plant height was obtained in T3 (20% seaweed extract application at once a week) compared to other treatments at 2 and 3 MAT (Fig.1).
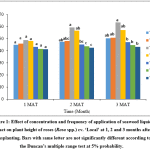 |
Figure 1: Effect of concentration and frequency of application of seaweed liquid extract on plant height of roses (Rosa spp.) cv. ‘Local’ at 1, 2 and 3 months after transplanting. Bars with same letter are not significantly different according to the Duncan’s multiple range test at 5% probability. Click here to View Figure |
Leaf Area
It was observed that there were significant (p<0.05) differences in the leaf area of rose plants grown under different treatments. Highest leaf area was recorded in T3 (20% seaweed extract application at once a week) compared to other treatments. Lowest leaf area was obtained in T7 (control) (Fig. 2).
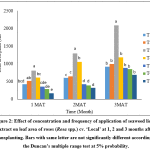 |
Figure 2: Effect of concentration and frequency of application of seaweed liquid extract on leaf area of roses (Rosa spp.) cv. ‘Local’ at 1, 2 and 3 months after transplanting. Bars with same letter are not significantly different according to the Duncan’s multiple range test at 5% probability. Click here to View Figure |
Plant Biomass
It was recorded that there were significant (p<0.05) differences in the plant biomass of rose plants between different treatments. Highest plant biomass was observed in T3 compared to other treatment as well as lowest plant biomass was obtained in T7 (Fig. 3).
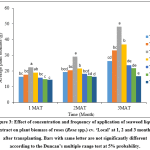 |
Figure 3: Effect of concentration and frequency of application of seaweed liquid extract on plant biomass of roses (Rosa spp.) cv. ‘Local’ at 1, 2 and 3 months after transplanting. Bars with same letter are not significantly different according to the Duncan’s multiple range test at 5% probability. Click here to View Figure |
Number of flowers
It was found that there were significant (p<0.05) differences in the number of flowers of Rose plants grown under different treatments at 1, 2 and 3 months after transplanting (MAT). Highest number of flowers was obtained in T3 compared to other treatments as well as lowest number of flowers was obtained in T7 at 1, 2 and 3 months after transplanting (Fig. 4).
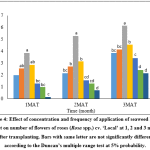 |
Figure 4: Effect of concentration and frequency of application of seaweed liquid extract on number of flowers of roses (Rosa spp.) cv. ‘Local’ at 1, 2 and 3 months after transplanting. Bars with same letter are not significantly different according to the Duncan’s multiple range test at 5% probability. Click here to View Figure |
Discussion
Plant Height
Highest plant height was observed in plants grown at T3 throughout the experiment. It could be due to increase in vegetative growth by the application of seaweed extract. Similar results were also reported in marigold,10 Cajanus cajan (L.)11 and Vigna sinensis L.12 Growth promoting substances in the seaweed extract could be the reason for the increased vegetative growth of crops.13 Further, the growth promoting potential is induced by the micro and macronutrients of seaweed extract.14
Materials which are applied in small quantities to promote the plant growth are called biostimulant.15 Sea weed extract is a biostimulant.16 Seaweed extract contains growth regulators (auxins, cytokinine and gibberellins), amino acids and mineral nutrients, which positively affect plant growth and cell division.17 Seaweed extracts improve nutrient uptake by roots.18 These activities in turn help to promote plant height. However, Rayorath et al.,19 opined that, application of seaweed extracts increased the plant growth at very low concentration. Therefore seaweed extract should be applied in optimum concentration to promote its stimulatory effects. This could be the reason for highest plant height observed in T3 throughout the experiment as the plants received optimum frequency and concentration of seaweed extract.
Plants grown at T1 and T2 would have received sub optimum amount of seaweed extract. Plants belong to these treatments had shown lowest plant height and reduced growth rate. Sutharsan et al.,8 reported that plant height in tomato was increased by the application of 20% seaweed liquid extract of Sargassum crassifolium compared to 10% of seaweed liquid extract.
Plants grown at T4, T5 and T6 would have received excess amount of seaweed extract. Increase in the frequency and concentration of application reduced the plant hieght.Seaweed extract is a biostimulant and biostimulatory effects would be expressed in low concentrations. Hence increase in the frequency and concentration of application had a negative effect on plant growth20. Seaweeds have plant growth regulators. In lower concentrations they promote plant growth. However in higher concentration they may suppress the growth. These could be the reasons for the reduction of plant height in these treatments.
Leaf Area
Application of seaweed liquid extract (SLE) increased the leaf area of experimental plants over the control. Several studies reported that seaweed extract has potential to increase leaf area of different plants. Mahajan21 reported that foliar application of seaweed extract increased leaf area of soybean (Glycine max L.) Shehata et al.,22 found that, application of seaweed extract increase the leaf weight of celeriac (Apium graveolens L.) plant. Srikrishnah and Sutharsan7 revealed that, foliar spray of seaweed extract increased the leaf number and leaf area of Anthurium (Anthurium andreanum L.).
Plants belong to T3 (20% seaweed extract application at once a week) would have received optimum amount of seaweed extract. It could be the reason for highest leaf area observed in this treatment. Sutharsan et al.,23 reported that, application of 20% sargassum crassifolium L. seaweed extract increased the leaf area of maize. Srikrishnah and Sutharsan7 reported that application of 20% Sargassum crassifolium L. extract increased the leaf number and leaf area of Anthurium plants. Sutharshan et al.,8 found that, maximum leaf number and leaf area produced by tomato (Lycopersicon esculentum L.) plants received 20% seaweed extract of Sargassum crassifolium L. Therefore it could be stated that, plants grown at T3 would have received optimum frequency and concentration of seaweed extract of Sargassum crassifolium L.
Plants grown at T3 had highest plant height. Plant height has a close relationship with leaf number and leaf area. Leaf area could be increased with plant height. It could also be the reason for highest leaf area observed in T3. Berntson and Weiner24 observed positive correlation between plant height and its total leaf area of Impatiens pallida plants.
Plants grown at T1 and T2 would have received reduced amount of seaweed extract to promote their growth. Seaweed extract is a biostimulant. It might promote growth at optimum concentration. Effect of five different concentrations of seaweed extracts (5%, 10%, 15%, 20%, 50% and 100%) of Sargassum crassifolium on maize (Zea mays L.) seedling performance was studied. In which, higher leaf area was observed at plants received 20% seaweed extract23. It showed that, seaweed extract promotes maximum growth at optimum concentration.
Plants grown at Treatment 4 to 6 would have received higher amount of seaweed extract than their requirement. At higher concentration seaweed extract might suppress the plant growth. Sutharshan et al.,8 found that application of higher concentration of seaweed extract reduced leaf number and leaf area in tomato (Lycopersicon esculentum L.) Average leaf area of maize was increased by 37.87% while applying 20% Sargassum crassifolium extract.23 This could be the possible reason for reduced LA observed in these treatments.
Plant Biomass
Plants grown at T3 would have received optimum frequency and concentration of seaweed extract. Plant biomass of Anthurium (Anthurium andreanum L.) was increased by foliar application of 20% of seaweed liquid extract.7 Sutharsan et al.,8 reported that shoot dry weight and root dry weight were increased by 80.92% and 81.57% respectively by the foliar application of 20% seaweed liquid extract of Sargassum crassifolium. Lingakumar et al.,25 also reported that seaweed extract enhanced the growth parameters and biomass accumulation in legumes. Plant biomass of maize (Zea mays L.) was also increased by the application of 20% seaweed liquid extract of Sargassum crassifolium.23 Further, Srikrishnah et al.,26 observed that, biomass production were in accordance with the trend of variances for LA in Dracaena. Hence, highest LA was observed in T3 in the experiment. These could be the reasons for the highest plant biomass production of plants at T3.
Plants grown at T1 and T2 would have received sub optimum amount of seaweed extract. It could be due to application of lowest concentration of seaweed extract. Seaweed acts as a biostimulant in lowest concentration. It should be applied in small quantity to enhance the growth rate.20 However, if the amount of application is less than the optimum, it could not promote growth. T4, T5 and T6 would have received excess amount of seaweed extract. Increase in the frequency and concentration of application reduced the plant biomass. Biostimulatory effects of seaweeds would be expressed in low concentrations. Hence increase in the frequency and concentration of application had a negative effect on plant growth.20 This could be the reason for reduction in plant biomass in these treatments.
Number of flowers
At 3 months after transplanting, highest number of flowers was obtained in T3 followed by T4 and lowest number of flowers was recorded in the T7. Potassium, Nitrogen, Magnesium and Phosphorous are the major macronutrients in seaweed. Further, there are some micronutrients in small quantity such as zinc, iron, manganese, and copper.8 Several studies reported that, seaweed could trigger flowering in many crops such as tomato,27 cluster beans12 and greengram.11 Poincelot28 also found that application of seaweed liquid extract of Ascopyllum nodosum increased flowering in tomato and broccoli.
Seaweed liquid extract of Sargassum crassifolium L. contains higher amount of potassium. Therefore all the treatments except control showed increase in flowers. Plants at T3 would have received optimum amount of seaweed extract. It could be the reason for highest number of flowers observed in this treatment. Several studies reported that application of 20% of seaweed liquid extract of Sargassum crassifolium L. could increase flowering. Sutharsan et al.,8 stated that, number of flowers on tomato was increased by 50.37% by the foliar application of 20% seaweed liquid extract of Sargassum crassifolium L. Srikrishnah and Sutharsan,7 found that foliar application of 20% seaweed liquid extract of Sargassum crassifolium L. could increase number of flowers in Anthurium. Sasikumar et al.,29 reported that 25% seaweed liquid extract increased flower number in Abelmoschus esculentus L.
Plants grown at T1 and T2 would have received sub optimum amount of seaweed extract to induce flowering. Lowest number of flowers was observed in T1 and T2 compared to T3 and T4. Sutharsan et al., 8 reported that 10% of seaweed liquid extract of Sargassum crassifolium L. reduced flowering compared to 20% concentration. Seaweed extract is a biostimulant. It might induce flowering at optimum concentration.
Plants grown at treatment 4 to 6 would have received higher amount of seaweed extract than their requirement. At higher concentration seaweed extract might suppress flowering. Stephenson30 found that lower concentrations of seaweed liquid extract accelerated growth than the higher concentrations of seaweed extract. Sutharshan et al., 8found that application of higher concentration of seaweed extract reduced flowering and number of flowers in tomato (Lycopersicon esculentum L.). In his experiment number of flowers was decreased by the application of 50% and 100% of seaweed liquid extract compared to 20% and 10% of seaweed extract. Sasikumar et al., 28 stated that low levels of seaweed liquid extract (12.5%, 25%) increased growth and flower number in Dictyota dichotoma compared to high levels (75%, 100%) of seaweed liquid extract. Hence increase in the frequency and concentration of application had a negative effect on plant growth20. Seaweeds have plant growth regulators. In lower concentrations they promote plant growth. However in higher concentration they may suppress the growth. These could be the reasons for the reduction of number of flowers in these treatments.
Conclusions
From the findings, it can be concluded that the application of 20%seaweed liquid extract of Sargassum crassifolium L.at one week interval could be the optimum concentration and frequency to increase the growth and flowering in rose plants. Lower (10%) and higher (30%) levels of application of seaweed extract of Sargassum crassifolium L. reduced the growth and flowering of rose plants.
Acknowledgements
The authors acknowledge the funding provided by Eastern University, Vantharumoolai, Sri Lanka.
Conflict of Interest
Authors declare no conflict of interest.
References
- Younis A., Riaz A., Aslam S., Ashan M., Tariq U., Javaid F., and Hameed M. Effect of different pruning dates on growth and flowering of Rosa. Pakistan Journal of Agriculture Science.2013; 50: 605-609.
- Krishnaraj S., A., and Wijesundara D., S., A. Cultivation of selected floriculture crops, National Botanic Garden, Peradeniya.2016.
- Gafoor A., Shaheen M., Iqbal M., Waseem K., and Nadeem M., A. Impact of various combination of NPK on the growth, yield, quality parameters of rose. Pakistan Journal of Agriculture Sciences.2000; 3: 1560-1562.
CrossRef - Azadi P., Khosh-khui M., Beyramizadeh E., and Bagheri H. Optimization of factors affecting in invitro proliferation and rooting of Rosa hybrid.International Journal of Agriculture Research.2007; 2: 626-631.
CrossRef - Blunden G., and Gordon S. Betaines and their sulphonio analogues in marine algae. Progress in Phycological Research.1986;4: 39-80.
- Hong D., Hein H., and Son P. Seaweedsfrom vietnam used for functional food,medicine and biofertolizer. Journal of Applied Phycology.2007; 19: 817-826.
CrossRef - Srikrishnah S., and Suthsarsan S. Effects of selected growth regulators and botanical extracts on the growth and flowering of Anthurium (Anthurium andreanum). Proceedings of Second International Research Symposium (pp. 43). Uwa Wellesa University, Sri Lanka.2018
- Sutharsan S., Nishanthini S., and Srikirshnah S.Effects of foliar applicationof seaweed (Sargassum crassifolium) liquid extract on the performance of Lycopersicon esculentum Mill in sandy regosol of Batticaloa district Sri Lanka. American-Eurasian Journal of Agriculture and Environment Sciences.2014b; 14: 1386-1396.
- Rama Rao K. Effect of seaweed extract on preparation, properties and use of liquid seaweed fertilizers from Sargassam work shop on algal products. Seaweed Research and Utilization Association.1990; 14: 7-8.
- Russo R., Poincelot R., P., and Berlyn G., P. The use of a commercial organic biostimulant for improved production of marigold cultivars. Journal of Home and Consumer Horticulture. 1993; 1:83-93.
CrossRef - Mohan V., R., VenkataramanKumarV., Murugeswari R., and Muthuswami S. Effect of crude and commercial seaweed extracts on seed germination and seedling growth in Cajanus cajan L. Phykos.1994; 33: 47-51.
- Sivasankari S.,Venkatesalu V., Anantharaj M., and Chandrasekaran M. Effects of seaweed extracts on the growth and biochemical constituents of Vigna sinensis. Bioresource Technology. 2006; 97: 1745-1751.
CrossRef - Blunden G. Agricultural uses of seaweeds and seaweed products, In: M. D. Guiry and G. Blunden, (Eds), Seaweed Resources in Europe: Uses and potential. Chichester, UK. 1991; 65-81.
- Rathore S., S., Chaudhary D.,R., Boricha G., N., Ghosh A., Bhatt B., P., Zodape S., T., and Patolia J., S. Effect of seaweed extract on the growth, yield and nutrient uptake of soybean (Glycine max) under rain fed conditions. South African Journal of Botany.2009; 75: 351-355.
CrossRef - Zhang X., and Schmidt R., E.The impact of growth regulators on the tacopherolα- status in water-stressed Poapratensis. International TurfgrassSociety Research Journal.1997; 8: 1364-1371.
- Khan W., Rayirath U.,P., Subramanian S., Jithesh M., N., Rayorath P., Hodges D.,M., and Prithiviraj B. Seaweed extracts as biostimulants of plant growth and development. Journal of Plant Growth Regulation.2009; 28: 386-399.
CrossRef - Berlyn G., P., and Rosso R. The use of organic biostimulant to promote root growth. Belowground Ecology.1990; 2:12-13.
- Crouch I., J., Smith M., T., Van Staden J., Lewis M., J., and Hoad G., V. Identification of auxins in commercial seaweed concentrates, Journal of Plant Physiology.1992; 139: 590-594.
CrossRef - Rayorath P., Khan W., Subramaniam S., Jithesh M., Rayirath U., Hodges D., and Craigie, J. Extracts as Biostimulants of plant growth and development. Journal of Plant Growth Regulation.2008; 4:386-399.
CrossRef - Mohanty D., Adikary S., P., and Chattopadhyay G., N. Seaweed liquid fertilizer and its role in agriculture productivity. International Quarterly Journal of Environmental Sciences.2013; 3: 23-26.
- Mahajan G., M. Effect of foliar application of seaweed extract and plant growth regulators on growth, productivity and quality of soybean (Glycine max L.) (Unpublished Doctoral dissertation, JNKVV), College of Agriculture, Jabalpur. 2014.
- Shehata S., M., Abdel-Azem, Abou El-Yazied A., and El-Gizawy A., M. Effect of foliar spraying with amino acids and seaweed extract on growth 57 chemical constitutes, yields and its quality of celeriac plant. European Journal of Scientific Research.2011; 58: 257-265.
- Sutharsan S., Nishanthini S., and Srikirshnah S. Effects of seaweed extract (Sargassum crassifolium) foliar application on seedling performance of Zea mays L., Proceedings of the International Symposium of Agriculture andEnvironmentRuhuna, Sri Lanka. 2014a; (pp. 119-122).
- Berntson G., M., and Weiner J. Size structure of populations within populations: leaf area and size in crowded and uncrowded Impatiens pallida individuals. Oecologia.1991; 85: 327-333.
CrossRef - Lingakumar K., Jeyaprakash R., Manimuthu C., and Haribaskar A. Gracilariaedulis– an effective alternative source as a growth regulator for Zea mays and Phaseolus mungo. Seaweed Research and Utilization.2002; 24: 117-123.
- Srikrishnah S., Sutharsan S., and SriyaniEdussuriyaPeiris. Effects of shade levels on leaf area and biomass production of three varieties of Dracenasanderiana L in the dry zone of Sri Lanka. Tropical Agricultural Research.2012; 23: 142-151.
CrossRef - Crouch I., and Van Staden J. Effects of seaweed concentrates on the establishment and yield of greenhouse tomato plants. Journal of Applied Phycology.1992; 4: 291-296.
CrossRef - Poincelot R., P.The use of a commercial organic biostimulant for field grown bedding plants. Journal of Home and Consumer Horticulture.1994; 1: 95-110.
CrossRef - Sasikumar K., Govindan T., and Anuradha C. Effect of seaweed liquid fertilizer of Dictyotadichotoma on growth and yield of Abelmoschus esculentus L. European Journal of Experimental Biology. 2011; 1:223-227.
- Stephenson W., A. Seaweeds in agriculture and horticulture, third ed., Rateover, Peruma valley, California.1974.


