Introduction
Chickpea is a crop that is environment friendly and sustains soil productivity. The benefits of the crop thus extend beyond the increase to the farmers and the farming systems. Chickpea has been well recognized as a valuable source of proteins particularly in the developing countries where majority of the populations depends on the low proceeds food for meeting the dietary requirements.1-3 Its magnitude of significance is more among Indians due to their reliance on vegetarians diet besides limited buying capacity of more than 200-250 millions (27%) people living below the poverty line (BPL). Like other pulses, supplementation of chickpea with cereals based diets is considered to be one of the possible solutions to the problems associated with protein energy malnutrition (PEM). The daily per capita availability of the chickpea is a source of approximately 2.3 % (56 Kcal) energy and 4.7 (27g) proteins to Indian population besides being a major source of calcium and iron.4
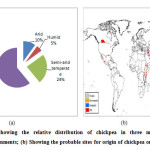 |
Figure 1(a): Showing the relative distribution of chickpea in three major kinds of environments; (b) Showing the probable sites for origin of chickpea on world map. |
The area occupied by the crop is 16% of the total pulse area but in some countries, e.g., India and Pakistan, it is the most important pulse crop and the area occupied could well be around 50% of the total pulse area.5 The productivity of chickpea is much low as compared to cereals crops due to varied reasons. Two of these are described here (i) excessive cold conditions adversely affect productivity, (ii) farmers are ignorant of precise doses of fertilizers recommended by the Agriculture Department for a particular cultivar and region, the techniques of cultivation of high yielding cultivars.6-7
Among the factors determining the desired yield of chickpea, use of quality seeds and yield stability of cultivars over varied environments are most critically important. The study of cultivar environment interaction provides useful information to identify stable cultivars over a range of environments. The growth and development of any individual plant species proceed at a speed and extent pre-determined by the genetic constitution. The eventual expression of the pattern, however, is modified by many interlocking environmental complexities which individual plant inhabitants. It is already have been stated that vigorous seed germinates rapidly and uniformly after planting and the emerged seedlings have the ability to grow vigorously under the wide range of environmental conditions.
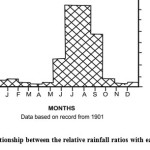 |
Figure 2: Annual relationship between the relative rainfall ratios with each month of year. |
However, with the release of new crop cultivars, it is imperative to work out the precise package of farm practices for each agroclimatic conditions so as to exploit their genetic potential fully as cultivars of the same species, differ in their inputs including nutrients.8 Most of the Indian soils supplementing chickpea are low in available P and S. As mentioned earlier, there is limitation on increasing the acreage for cultivation, it is, therefore, highly logical to innovate ways that can improve the productivity as well as environment sustainability. In this regard, an approach could be to make plants utilize fully the available resources leading to maximum harvesting of solar energy and subsequently enhancing the active sites but without interfere with better N2-fixation efficiency. To attain such goal, the use of GA3 may play various important roles.9-11 Moreover, enhancing P and S availability to chickpea along with gibberellic acid (GA3) holds promise in the present scenario of escalating prices of phosphatic and sulphatic fertilizers and a general deficiency of P in Indian soils.12-13 After the determination of the promising responses of chickpea to P, S or GA3 or combined application in terms of maximum yield, protein value and economic profitability of production and environmental resilience (better N-fixation) in a particular environment as well as a very little investigation is in the existing literature, the present study was undertaken.
Materials and Methods
The soil sample was analysed in the Soil Testing Laboratory, Government Agriculture Farm, Quarsi, Aligarh for various physico-chemical properties. The physico-chemical analysis of the mixture of soil and FYM used for filling of the pots is as; texture, sandy loam; pH, 8.04; E.C (dSm-1), 0.65; N-P-K (Kg/ha), 210-34.32-209.20 respectively and calcium carbonate (%), 0.10. Aligarh district has the same soil composition and the appearances as those found generally in the plains of Northern India. The average temperatures for December and January are about 15˚C and 13˚C respectively.
 |
Figure 3: Annual relationship between the relative temperature ratios with each month of the year. Click here to View figure |
Besides this, the average rainfall is 847.3 mm. More than 85% of the total rainfall occurs during June to September. The relative humidity of the winter season ranges between 56% to 77% with an average 66.5% that of the summer between 37% and 49% with an average of 43% and that of the monsoon seasons, between 63% and 73% with an average of 68%. Subsequently, seeds were inoculated with the recommended strain of Rhizobium and then were sown. The amount of GA3 was dissolved in 10 Ml ethyl alcohol and the final volume was made 100 Ml using DDW. Further dilutions of the stock solutions (10-6M GA3) were made with DDW. A uniform recommended basal dose of 40 kg N + 30 kg P2O5/ha (17.9 mg N+13.4 mg P/kg soil) was applied to all pots with the half dose of N and full dose of P giving at the time of sowing and the remaining half dose of N after 30 DAS with the help of Pora.
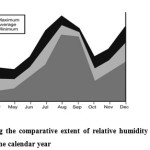 |
Figure 4: Figure showing the comparative extent of relative humidity of environment at the each moth of the calendar year. Click here to View figure |
The remaining amount of N dose was compensated with urea. Finally, four plants per pot were maintained. A water-sprayed control was also included in the scheme of treatments. The experiment was performed according to a simple randomized design.
Sampling Techniques
One plant from each replicate was uprooted randomly at the various sampling stages to assess the performance of the crop on the basis of growth and biochemical parameters at 90 and 100 DAS while yield associated attributes at harvest.
Growth Characteristics
Length of shoot on per plant basis was determined with the help of a metre scale. LAI is determined by the following formula suggested by Watson 14:
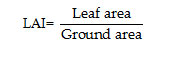
At the flowering stage, sample plants were collected from 1-2 cm linearly for the collection of nodules. The roots of the collected samples were washed carefully and all the nodules were separated manually for counting their number and later oven dried at 70˚C for 72 hours to record the dry weight of the nodules.
Biochemical Characteristics
The NR and N-ase activities in fresh leaves were estimated by the methods of Jaworski15 and acetylene reduction assays method of Turner and Gibson16 respectively. The Lb content in fresh nodules was estimated following the method of Sadasivam and Manickam.17 The Lb content was calculated by the following formula:
Where, D is the initial dilution. A556 and A539 are the absorbances at 556 and 539 nm, respectively. The calculation is based upon the equation E = 23.4 x 103 mol-1cm-1. N and P were estimated according to the methods of Lindner18 and Fiske and Subbarow19 respectively whereas K was estimated flame photometrically.
Yield Characteristics
Number of pods per plant was determined at physiological maturity from two remaining plants. The pods were manually removed from all the harvested plants and number of pods per plant determined. Pod weight per plant was estimated with the help of electronic balance. The total seeds of two plants were threshed, cleaned and allowed to dry in the sun for some time and their weight was obtained with the help of an electronic balance, with expressing their weight on per plant basis (seed yield per plant). The total protein content in the dry seeds was estimated by adopting the methodology of Lowry.20
Data Collection and Analysis
All data were analyzed statistically adopting the analysis of variance technique, according to Gomez and Gomez.21 In applying the F test, the error due to replicates was also determined. When ‘F’ value was found to be significant at 5% level of probability, critical difference (CD) was calculated. Mean differences among the treatments were adjusted by using the Ducan multiple range test (DMRT) at 5% level of significance also.
Results
Among treatments, combination of pre-sowing seed treatment with GA and foliar spray of GA, P and S (SGA+ FGAPS) gave the maximum value for shoot length per plant at both sampling stages (90 and 100 DAS). Its effect was, however, equal to that of SGA+ FGAS at each sampling stage. Treatment SGA+ FGAPS gave 86.71 and 78.95% higher value at 90 and 100 DAS respectively than the water-sprayed control, i.e. FW (Table 1). Treatment SGA+ FGAPS gave the maximum value of LAI at both sampling stages. Its effect was followed by that of SGA+ FGAP and SGA+ FGAS at each stage of sampling and also by that of SGA+ FGA at 90 DAS. Treatment SGA+ FGAPS gave 218.42 and 186.21% higher value at 90 and 100 DAS respectively than FW (Table 1).
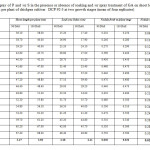 |
Table 1: Effect of spray of P and /or S in the presence or absence of soaking and /or spray treatment of GA on shoot length, LAI, nodule fresh and dry weight per plant of chickpea cultivar DCP 92-3 at two growth stages (mean of four replicates). |
Treatment SGA+ FGAPS gave the maximum value of nodule number per plant at each sampling stage. Its effect was followed by that of SGA+FGAS and SGA+FGAP at 90 DAS and was, however, equal to that of SGA+ FGAS at 100 DAS. Treatment SGA+ FGAPS gave 136.84 and 145.00% higher value at 90 and 100 DAS respectively than FW (Fig. 5a). Treatment SGA+FGAPS gave the maximum value of nodule fresh weight per plant at each sampling stage. Its effect was, however, equal to that of SGA+ FGAS at both sampling stages. Treatment SGA+ FGAPS gave 161.90 and 136.00% higher value at 90 and 100 DAS respectively than FW (Table 1). Treatment SGA + FGAPS gave the maximum value of nodule dry weight per plant at each sampling stage. Its effect was, however, equal to that of SGA+ FGAS at 90 DAS and was followed by that of the same treatment at 100 DAS. Treatment SGA+ FGAPS gave 233.34 and 250.00% higher value at 90 and 100 DAS respectively than FW (Table 1).
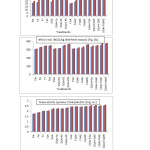 |
Figure 5: Effect of spray of P and / or S in the presence or absence of soaking and / or spray treatment of GA3 on (a) nitrate reductase activity (b) nodule number per plant (c) N-ase activity of cultivar DCP 92-3 of chickpea. |
Treatment SGA+ FGAPS gave the maximum value of NRA at each sampling stage. Its effect was, however, equalled by that of SGA+ FPS, FGAPS, SGA+ FGAS at both stages and was also by that of SGA+ FS at 100 DAS. Treatment SGA+ FGAPS gave 22.37 and 22.46 % higher value at 90 and 100 DAS respectively than FW (Fig. 5b). Treatments SGA+ FGAPS and SGA+ FGAS were at par in their effect and gave maximum value of N-ase at each stage of sampling. Treatments, FGAPS, SGA+ FGAP and FGAS being at par occupied second position at each stage and treatments FGAP, SGA+ FPS and FGAS also at 90 DAS. Treatment SGA+ FGAPS gave 206.13 and 215.38% higher value at 90 and 100 DAS respectively than FW (Fig.5c). The effect of treatments on N content was not found significant at both stages of sampling (Table 2). Treatment SGA+ FGAPS gave the maximum value of P content at each sampling stage. Its effect was, however, equal to that of FGAPS at both sampling stages and also to that of SGA+ FGAS at 90 DAS.
Treatment SGA+ FGAPS gave 37.50 and 41.46% higher value at 90 and 100 DAS respectively than FW (Table 2). The effect of treatments on K content was not found significant at both stages of sampling (Table 2). Treatment SGA+ FGAPS gave the maximum value of nodule Lb content at each sampling stage. Its effect was, however, equal to that of FGAPS at both sampling stages and also to that of SGA+ FGAS at 90 DAS. Treatment SGA+ FGAPS gave 17.50 and 11.46% higher value at 90 and 100 DAS respectively than FW (Table 2).
Table 2: Effect of spray of P and /or S in the presence or absence of soaking and /or spray treatment of GA on leaf N-P-K and Lb content per plant of chickpea cultivar DCP 92-3 at two growth stages (mean of four replicates).
|
Treatments |
N-content |
P – content |
K-content |
Leghaemoglobin content /plant |
||||
|
90 DAS |
100 DAS |
90 DAS |
90 DAS |
90 DAS |
100 DAS |
90 DAS |
100 DAS |
|
| FW |
3.200 |
3.210 |
39.50 |
41.50 |
3.420 |
3.430 |
83.33 |
84.83 |
| FP |
3.240 |
3.250 |
39.83 |
46.83 |
3.450 |
3.480 |
89.00 |
90.86 |
| FS |
3.270 |
3.310 |
41.00 |
45.66 |
3.470 |
3.490 |
93.83 |
95.12 |
| FPS |
3.340 |
3.390 |
45.66 |
47.88 |
3.490 |
3.500 |
94.47 |
96.41 |
| SGA |
3.750 |
3.770 |
56.33 |
57.00 |
3.660 |
3.700 |
84.00 |
85.88 |
| SGA+FP |
3.790 |
3.810 |
57.33 |
59.66 |
3.720 |
3.740 |
85.94 |
87.05 |
| SGA+FS |
3.800 |
3.870 |
58.33 |
60.50 |
3.750 |
3.780 |
94.80 |
97.27 |
| SGA+FPS |
3.810 |
3.820 |
63.00 |
65.33 |
3.810 |
3.830 |
99.22 |
100.94 |
| FGA |
3.450 |
3.520 |
59.50 |
60.00 |
3.710 |
3.760 |
84.47 |
85.33 |
| FGAP |
3.510 |
3.640 |
60.50 |
60.83 |
3.740 |
3.800 |
85.27 |
88.72 |
| FGAS |
3.720 |
3.740 |
62.33 |
62.83 |
3.790 |
3.810 |
88.13 |
91.72 |
| FGAPS |
3.840 |
3.860 |
64.00 |
65.00 |
3.800 |
3.840 |
98.86 |
99.41 |
| SGA+FGA |
3.510 |
3.790 |
61.83 |
62.66 |
3.860 |
3.890 |
90.50 |
94.77 |
| SGA+FGAP |
3.780 |
3.810 |
63.16 |
63.33 |
3.920 |
3.970 |
94.50 |
96.50 |
| SGA+FGAS |
3.790 |
3.840 |
63.16 |
64.33 |
3.950 |
3.980 |
96.44 |
102.88 |
| SGA+FGAPS |
3.900 |
4.000 |
64.16 |
65.66 |
3.970 |
3.990 |
101.97 |
103.88 |
| C.D. at 5% |
NS |
NS |
3.933 |
NS |
0.26 |
0.26 |
6.51 |
6.66 |
Treatment SGA+ FGAPS gave the maximum value for pod number per plant. Its effect was, however, equal to that of SGA+ FGAS. Treatment SGA+ FGAPS gave 121.72% higher value than FW (Table 3). Treatment SGA+ FGAPS exhibited the maximum value for seed weight per plant. Its effect was, followed by that of SGA+ FGAS. Treatment SGA+ FGAPS gave 200% higher value than FW (Table 3). Treatment SGA+ FGAPS gave the maximum value of seed number per pod. Its effect was, however, equal to that of SGA+ FGAS, SGA+ FGAP and SGA+ FGA. Treatment SGA+ FGAPS gave 86.14% higher value than FW (Table 3). Treatment SGA+ FGAPS gave the maximum value of seed yield per plant. Its effect was, however, equal to that of FGAPS, FGA, SGA+ FGA, SGA+ FGAS, FGAS, SGA+ FGAP, SGA+ FS, SGA+ FP, FGAP, SGA and FPS. Treatment SGA+ FGAPS gave 20.59 % higher value than FW (Fig. 6a). Treatment SGA+ FGAPS gave the maximum value of seed protein content. Its effect was, however, equal to that of SGA+ FGAS and SGA+ FGAP. Treatment SGA+ FGAPS gave 16.11% higher value than FW (Fig. 6b).
 |
Figure 6 (a&b): Effect of spray of P and / or S in the presence or absence of soaking and / or spray treatment of GA3 on seed yield and protein content of cultivar DCP 92-3 of chickpea. Click here to View figure |
Table 3: Effect of spray of P and/ or S in the presence or absence of soaking and/or spray treatment of GA on pod number, pod weight and seed number per plant of chickpea cultivar DCP 92-3 at harvest (mean of four replicates)
|
Treatments |
Pod number/plant |
Pod weight/plant |
Seed number/plant |
|
|
120 DAS |
120 DAS |
120 DAS |
||
| FW |
15.20 |
34.20 |
1.00 |
|
| FP |
17.40 |
35.29 |
1.25 |
|
| FS |
18.25 |
35.37 |
1.50 |
|
| FPS |
20.70 |
36.22 |
1.75 |
|
| SGA |
24.60 |
37.90 |
1.75 |
|
| SGA+FP |
25.10 |
34.23 |
2.00 |
|
| SGA+FS |
28.90 |
38.22 |
2.00 |
|
| SGA+FPS |
27.40 |
39.76 |
2.00 |
|
| FGA |
27.40 |
39.11 |
1.50 |
|
| FGAP |
29.50 |
38.00 |
1.75 |
|
| FGAS |
30.20 |
35.90 |
2.00 |
|
| FGAPS |
30.70 |
35.30 |
2.25 |
|
| SGA+FGA |
28.90 |
40.00 |
2.00 |
|
| SGA+FGAP |
29.10 |
4.90 |
2.50 |
|
| SGA+FGAS |
33.40 |
38.74 |
2.75 |
|
| SGA+FGAPS |
33.70 |
43.87 |
3.00 |
|
| C.D. at 5% |
1.85 |
2.33 |
0.14 |
|
Discussion
The improvement of a crop depends to a larger extent on the genetic variability as it provides us the raw materials for selection of better genotypes. Little natural variability is found in chickpea for conspicuous morphological and physiological characters. Overwhelmingly, GA3 serve manifold growth related functions in plants by enhancing replication, transcription and different enzymatic systems.22-24 An increase in growth parameters like shoot and root lengths, fresh and dry weights in plants treated with GA3 in accordance with the known fact that exogenous application of PGRs evoke the intrinsic genetic potential of the plant causing increase in elongation of inter nodes as a consequence of cell division and cell wall extensibility. Enhancement in leaf-nutrients, particularly N due to GA3 application could be attributed to the compositional or chemical change in plants leading to alterations in N concentration. Acquisition and assimilation of N is a fundamental process that is essential for the growth and development of plants. N is available to plants mainly in the form of nitrate (NO3). The NO3 taken up by the plants is first reduced to NH3 by the enzyme namely, nitrate reduction (NR) and nitrite reductase (NiR). N assimilating enzymes (NR and N-ase) which are crucial for plant growth and also provide effective targets for herbicide development also. This enhancement could be the results of increased uptake of nutrients, enhanced photosynthesis and improved trans location of photosynthates and other metabolites to the reproductive parts. This sustained increase in the N-P-K contents of the treated plants which is expected to culminate the maximization of the seed yield and seed protein content.25 Needless to say, GA3 might be involved in formation of seeds in pods and their optimum nourishments have resulted in less number of aborted seeds and thus maximized the survival of fertile seeds/pods.
The growth improving effect of pre-sowing seed treatment for 8 h and foliar treatment at 60 and 70 DAS with 10-6M GA3 over their respective water treated control on plant height and LAI of chickpea grown with a dose of N and P could be explained on the basis of its roles and the fact that the supply of GA3 by pre-sowing seed treatment or by foliar application would more than compensate the inadequate level of GA3 to growing crops. Also, GA3 foliar spray increased plant height and leaf length. An increase in growth parameters like shoot length in plants sprayed with GA3 in accordance with the known fact that exogenous application of PGRs evoke the intrinsic genetic potential of the plant causing increase in elongation of inter nodes as a consequence of cell division and cell wall extensibility.26-28
The improving effect of the spray of a small quantity of P and /or S alone or in combination with the soaking and/or foliar spray treatment of GA3 over the respective control on the growth parameters of chickpea is a noteworthy observation. The promoting effect of P and S on the growth parameters can be traced to their various roles. Thus, being important essential nutrients, P and S are directly or indirectly involved in growth of chickpea like other crops through the production of metabolic compounds. These metabolites, in turn, encourage the formation and enlargement of new cells in treated plants, hence the increased height of plants, extra leaf formation and larger LAI. It may be added that these results on the improving effect of foliar application of P and S broadly corroborate the earlier findings of Naqvi29 and Khan.30 Improvement in shoot length and LAI would have contributed in improving the ability of treated plants for nodule and biomass production.
The augmenting effect of leaf-applied GA3 over the water-sprayed control as also its superior effect on N-ase and NR activities and leaf NPK content is worth mentioning. The increase in NR and N-ase activities can be attributes to the hormone-induced increase in transcription and/or translation of the gene that codes for NR and N-ase as well as in NPK content to its role in enhancing the permeability of membranes and absorption of nutrients.31 These results are also in accordance with the data of earlier workers including Premabatidevi,32 on NR activity; and Hassanpouraghdam33 on N-ase and NPK content. Enhancement in leaf-nutrients, particularly N due to GA3 application could be attributed to the compositional or chemical change in plants leading to alterations in N concentration.33
The enhancing effect of pre-sowing seed treatment for 8 h and foliar treatment at 60 and 70 DAS with 10-6M GA over their respective water treated control NR and N-ase activities and nutrient contents of chickpea grown with the recommended basal dose of N and P is a noteworthy observation. This may also be attributed, as for growth characters, to its roles on one hand and compensation of the ‘hidden hunger’ for GA by its pre-sowing seed treatment or foliar application on the other. The augmenting effect of foliar spray of P and /or S alone or in combination with the soaking and /or foliar spray treatment of GA over the respective control on NR and N-ase activities and N, P and K content of chickpea grown with a basal dose of N and P is not far to seek. P and S being component of the various metabolites involve in the production of organic compounds which, in turn, encourage the formation and proper supply of proteins to be involved in the formation of enzymes. Hence higher activities of NR and N-ase in treated plants. The higher values for N, P and K content in leaves of P and /or S sprayed plants may be due to the stimulatory effect of leaf-applied P and S on absorption of nutrients from the soil.34 Increased NR and N-ase activities might be responsible for increasing biosynthesis of protein of treated plants.
Moreover, improvement in N, P and K content would also have enhanced content of protein on one hand and Lb content on the other. Furthermore, GA3 contributes towards enhancing the capacity of the treated plants for biomass production as reflected in shoot and root dry weight of the plants. This enhancement could be the results of increased uptake of nutrients, enhanced photosynthesis and improved translocation of photosynthates and other metabolites to the reproductive parts. This sustained increase in the above mentioned parameters of the treated plants which is expected to culminate the maximization of seed yield and seed protein content.
The increase in the number of pods per plant and seed number per pod weight resulting from the foliar application of GA3 in comparison with the water-sprayed control as well as its superior effect applied at 10-6 M on these parameters of chickpea receiving the recommended basal dose of N and P is worth mentioning. The increase in these yield attributes may be traced to its various role leading to observed higher values for growth characters and, physiological and biochemical parameters of treated plants.35-38
The augmenting effect of pre-sowing seed treatment with 10-6 M GA for 8 h over water-soaking treatment on pods per plant and seeds per pod and of spray treatment at 60 and 70 DAS with the same concentration of GA3 in comparison with the water-sprayed control on these parameters is understandable. The augmenting effect of foliar treatment with 10-6M GA for 60-70 DAS over water-spray treatment on seeds per pod is understandable. In addition, GA3 might have increased the translocation of assimilates to the reproductive organs which resulted in the maximum number of pods per plant up to a certain levels of GA3 application39. Instinctly like other growth regulators, GA3 might be involved in formation of seeds in pods and their optimum nourishments have resulted in less number of aborted seeds and thus maximized the survival of fertile seeds/pods in chickpea. Lastly, Makarem and Mokhtar40 reported that GA3 could lead to an increase in fruit set of deciduous trees and played a major role in enlarging fruit size. Similarly, El-Seginy and Khalil41 reported also that GA3 application increased fruit set, fruit weight, and as a result increased the yield.
The improvement in pods per plant, seeds per pod, pod and seed weight per plant of chickpea grown with the recommended basal dose of N and P, due to foliar application of a small quantity of P and /or S alone or in combination with the soaking and /or spray of GA3 over the respective control is not far to seek. Also, P and S influence differentiation in plants.42 The improvement in growth, physiological and biochemical parameters resulted from the spray of these nutrients together with enhancement in differentiation may lead to the improvement in pods per plant, seeds per pod43 on foliar application of P and S. The increased yield attributing parameters of treated plants, particularly pods per plant is likely to have contributed to the improved seed yield. The observed increase in seed protein content due to foliar application and pre-sowing seed treatment of GA3 is not surprising. An improvement in protein synthesis may result from the application of GA3 and P and S,32, 43 hence higher values for seed protein content on P and S although on basal application.40-41
References
- Khan, F, Mazid, M., Khan, T.A., Quddasi, S, Roychowdhury, R., and Naqvi N. Application of Sesquiterpene (GA3) to spermology: A contradictory report. Res. J. Biol. 1: 45-51 ,(2013).
- Naqvi, N. Khan, T. A., Mazid, M, Khan, F, Quddasi, S, and Roychowdhury, R.. Phytoremediatory potential of Guava and Ashok tree at three different sites of Bareilly district-A case study: ARPN J. Agric. and Biol. Sci. 9(3): 101-109, (2014).
- Quddasi, S, Khan, F, Mazid, M., Khan, T. A. Shakeel. A. and Arshad, M. (2014). Quality status of nutrients, vitamins and hormones under pathological threats in tomato: Basic scenario. Medi. Chemis. Anal. 4(1):12-21, (2014).
- Sikarwar, V. S. Evaluation of chickpea (Cicer arietinum L.) germplasm in relation to different growth habits to develop selection criteria for high seed yield. M.Sc. (Ag.). Thesis, IGAU, Raipur, (2004).
- Dar, K. and Williaim, D. Role of chickpea in grey to green revolution. Inaugural Address at the International Chickpea Congress 2003, 20-22 January 2003, Raipur, Chhattisgarh, India, 5P. (2003).
- Patil, S.V., Halikatti, S. I., Hiremath, S.M., Babalad, H.B., Sreenivasa, M. N., Hebsur, N.S. and Somanagouda, G. Effect of organic manures and rock phosphate on growth and yield of chickpea (Cicer arietinum L.) in vertisols. Karnataka J. Agric. Sci. 24: 636-638,(2011).
- Mazid, M. and Mohammad, F. Environmental constraints responsible for reduction in productivity of chickpea: Revisit an old problem. Int. J. Envt. Eng. Manage. 3 (5): 32-35, (2012).
- Langer, R. H. M. Mineral nutrition of grasses and cereals. In: The Growth of Cereals and Grasses. F. L. Milthorpe and J. D Ivins (eds.). Butterworths, London, pp. 213-226, (1966).
- Gana, A.S. The role of synthetic growth hormones in crop multiplication and improvement. African J. Biotechnol., 10:10330-10334 (2010).
- Mazid, M., Ali, B., Hayat, S. and Ahmad, A. The effect of 4-chloro-3-indoleacetic acid on some growth parameters in mung bean under cadmium stress. Turk. J. Biol., 34: 9-13(2010).
- Khan, T. A., Mazid, M., and Mohammad, F. Sulphur management: An agronomic and transgenic approach. J. Indus. Res. Technol., 1 (2): 147-161, (2011a).
- Mazid, M., Khan, T. A., and Mohammad, F. Cytokinins, A classical multifaceted hormone in plant system. J. Stress Physiol Biochem., 7 (4): 347-368 (2011b).
- Khan, T. A., and Mazid, M. Nutritional significance of sulphur in pulse cropping system. Biol. Med., 3 (2):114-133, (2011).
- Watson, D.J. The dependence of net assimilation rate on leaf area index. Ann. Bot., 22: 37-54 (1958).
- Jaworski, E.G. Nitrate reductase assay in intact plant tissues. Biochem. Biophys. Res. Commun., 43: 1274-1279, (1971).
- Turner GL, Gibson AH. In: Bergersen FJ, editor. Methods for evaluating biological nitrogen fixation. New York: John Willey & Sons. p. 111-138 (1980).
- Sadasivan, S. and Manickam, A. Biochemical Methods. 3ed Ed., New Age International, New Delhi, (2008).
- Lindner, R.C. Rapid analytical methods for some of the more common inorganic constituents of plant tissues. Plant Physiol., 19: 76-89, (1944).
- Fiske, C.H. and Subbarow, Y. The colorimetric determination of phosphorus. J. Biol. Chem., 66: 375-400, (1925).
- Lowry, O.H., Rosebrough, N.J., Farr, A.L. and Randall, R. J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193: 265-275, (1951).
- Gomez, K.A. and Gomez, A.A.. Statistical Procedures for Agricultural Research. 2nd Ed. J. Wiley and Sons, New York , (1984).
- Mazid, M., Khan, T. A., and Mohammad, F. Response of crop plants under sulphur stress tolerance: A holistic approach. J. Stress Physio. Biochem., 7 (3): 23-57, (2011b).
- Khan, T. A., Mazid, M., & Mohammad, F. Climate change, sustainable agriculture and future needs: A perspective of parallel re-thinking. J. Indus. Res. Technol., 1(2): 40-50, (2011b).
- Khan, T. A., and Mazid, M. Nutritional significance of sulphur in pulse cropping system. Biol. Med., 3 (2): 114-133, (2011).
- Mazid, M. Application of spray and seed priming GA3 with P and S ameliorate seed protein content by augmenting photosynthetic attributes, enzymes activities and leghemoglobin content of chickpea. International J. Basic App. Bio., 1(2):14-19, (2014a).
- Thakare, U., Patil, N. and Malpathak, N. Performance of chickpea under the influence of gibberellic acid and oxygenated peptone during germination. Adv. Biosci. Biotechnol. 2: 40-45: (2011).
- Mazid, M., Khan, T.A., Mohammad,, F. Role of nitrate reductase in nitrogen fixation under photosynthetic regulation. World. J. Pharmaceu. Res. 1(3): 386-414, (2012a).
- Mazid, M. Seed priming application of gibberellic acid on growth, biochemical, yield and protein status of Chickpea (Cicer arietinum L. cv. DCP 92-3). Int. J. Genetic Eng. Biotech., 5(1): 17-22, (2014b).
- Naqvi, A., Afridi, M.M.R.K. and Samiullah. Effect of phosphorus and sulphur on the growth and yield of Laha -101. Geobios., 4: 253-254, (1977).
- Khan, T. Studies on the Effect of Soil and Foliar Application of Nutrients on the Performance of Mustard. Ph.D. thesis, Aligarh Muslim University, Aligarh, India, (1993).
- Taiz, L. and Zeiger. E. Plant Physiology. 5th Ed. Sinauer Associates, Inc., Sunderland, MA, USA, (2010).
- Khan, T. A, Mazid, M and Quddasi, S. Role of organic and inorganic chemicals in plant-stress mitigation. Approaches to Plant Stress and their Management (Ed.).P. K. Gaur and P. Sharma. DOI-10.1007/978-81-322-1620-9-3. Springer, India, (2014). Pp. 39-52.
- Premabatidevi, R.K. Effect of IAA, GA3 and kinetin on nitrate reductase and nitrite reductase in the leaves of a tree legume (Perkia javanica Merr). Indian J. Plant Physiol., 3: 97-101, (1988).
- Hassanpouraghdam, M.B., Hajisamadi, A. B. and Khalighi, A. Gibberellic acid foliar application influences growth, volatile oil and some physiological characteristics of lavender (Lavandula officinalis Chaix). Romanian Biotechnol. Letters, 16: 6322-6327, (2011).
- Mohammad, F. Phosphorus application improves physiological parameters growth and yield of mustard (Brassica juncea L.). J. Indian bot. Soc. 83: 42-45, (2000).
- Estruch, J.J., Pereto, J.G., Vercher, Y. and Beltran, J.P. Sucrose loading in isolated veins of Pisum sativum: Regulation by abscisic acid, gibberellic acid and cell turgor. Plant Physiol., 91: 259-265, (1989).
- Akter, A., Ali, E., Islam, M.M.Z., Karim, R. and Razzaque, A.H.M. Effect of GA3 on growth and yield of mustard. Int. J. Sustain. Crop Prod., 2(2): 16-20 (2007).
- Uddin, M. M., Samad, A., Khan, M.R. Begum, S. and Salam, M.A. Effect of sowing dates on the yield and some of its components of mustard and rape-seed. Bangladesh. J. Sci. Ind. Res., 21(1-4): 160-165, (1986).
- Makarem, M. M. and H. Mokhtar. Effect of Bio-enzyme and gibberellic acid on fruit set, yield and fruit quality of Anna apple trees. J. Agric. Sci., Mansoura Uni. 21(7): 2661-2669, (1996).
- El-Seginy, A.M. and B.M. Khalil. Effect of spraying some nutrients and gibberellic acid on leaf mineral content, fruit characters and yield of Le-Conte pear tress. J. Agric. Sci., Mansoura Univ. 25 (6): 3529-3539, (2000).
- Mozer, T.J. Control of protein synthesis in barley aleurone layers by the plant hormones gibberellic acid and abscissic acid. Cell, 20: 479-485, (1980).
- Mazid, M. Khan, T.A., Mohammad, F. Nooris, N. Physico-biochemical effects of pre-sowing seed treatment with gibberellic acid on Helianthus annus L.- National conference of Plant Physiology” Current Trends in Plant Biology Research”: 895-896. Junagarh, Gujarat, (2013).
- Mohammad, F., Samiullah and Afridi, M.M.R.K. Effect of phosphorus and sulphur spray on mustard yield. Geobios. 13: 8‐12, (1986).
- Mazid, M and Naqvi, N. Differential Yield and Quality Response of Four Chickpea Cultivars Following the Foliar Spray of Five Selected Plant Growth Regulators. Agriculture Science Digest. , 34 (4): 268-272, (2014).
- Mazid, M and Nooris, N. Key Approaches of Plant Growth Regulator Application in Searching of Best Time for Enhancing Nitrogen Fixation Capacity of Chickpea Cultivar DCP 92-3. Unique Journal of Ayurvedic and Herbal Medicines. 2(5): 8-18, (2014).
- Mazid, M and Roy chowdhury, R. Leaf N-P-K Content As Indicators of Yield, Total Protein and Sugar Content of Seeds of Bengal Gram (Cicer Arietinum L.). Unique Journal of Pharmaceutical and Biological Sciences. 2(5): 23-30, (2014).
