Introduction
By altering its metabolism, growth and development, abiotic stresses can directly or indirectly affect the physiological status of an organism1,2 and adversely affect agricultural productivity.3 Salinity is the most destructive factor among the abiotic stresses, which considerably limits the productivity of crops. Salinity has affected a large area of land in the world which is increasing day by day and it is the more prominent problem in irrigated crop fields. Pitman and Lauchli4 estimated that at least 20% of total irrigated lands in the world are salt-affected. The anthropogenic activities resulted in secondary salinity that disrupt the hydrologic balance of the soil between water applied (irrigation or rainfall) and water used by crops (transpiration).5
The ability of plants to utilize water is reduced due to salinity thus, resulted in reduced growth rate and also change metabolic processes in plants.6,7 In addition, it decreases plant growth and yield depending on the plant species, salinity levels and ionic composition of salt.8 Seed germination, seedling growth, vigour, vegetative growth, flowering and fruit set are adversely affected by high salt stress, ultimately causing diminished economic yield and also quality of product. Major organic osmolytes such as protein and proline accumulated naturally in many plant species when subjected to different abiotic stresses.9 These compounds play adaptive role in mediating osmotic adjustment and protecting sub cellular structures in stressed plants.
Salt stress is reported as a serious problem.10 Salinity decreases crop productivity and threatens the global food balance11. It is present in soluble form from low level to high level in soil atmosphere and resulted in decreased water uptake both during imbibition and seedling establishment followed by uptake of ions.12,13 Throughout the world, salinity stress negatively impacts agricultural yield affecting production whether it is for subsistence or economic gain.14 The plant response to salinity consists of numerous processes that must function in coordination to alleviate both cellular hyper osmolarity and ion disequilibrium. In addition, crop plant must be capable of satisfactory biomass production in a saline environment.15,16
The genus Vigna L. includes many wild and cultivated species with pantropical distribution.17 Among the pulse crops (i.e. annual leguminous food crops) Vigna is far the most important. For mankind Vigna species are important sources of high quality proteins and amino acids and like many other leguminous plants; they play a key role in crop rotation due to their ability to fix nitrogen. To support the awareness on this matter; the United Nations declared 2016 the International year of pulses. Salinity stress is one of the main factors limiting legume productivity in many parts of the world.18 In this study, an attempt was made to examine the effect of salinization on four species of Vigna viz. V. mungo (urd), V. angularis (rais), V. radiata (moong) and V. aconitifolia (moth) through glasshouse experiment. The main aim of this study was to compare salt (NaCl) stress tolerance potential of selected Vigna spp.
Material and Methods
In this study two salt stress levels (50 mM (S1) and 100 mM (S2)) were prepared using NaCl. In addition one control (0 mM (C)) was also maintained.
Experiment
Seeds of selected Vigna species viz. Vigna aconitifolia, V. angularis, V. mungo and V. radiata commonly grown in Kumaun Himalayan region were collected from the native market of Nainital. Healthy and uniform seeds of all species were surface sterilized and washed with distilled water. The seeds were placed in pots with sterile soil and about 10ml distilled water for control or the respective solution was poured in every pot. There were 3 seeds per species in one pot and a total of 15 seeds were shown for creating replicate of one treatment. Germination test were conducted under condition of 12 hours light/dark cycle with 11ºC minimum and 36oC maximum temperature in the glasshouse of Botany Department, Kumaun University Nainital. Different concentration of salt solution was added gradually to pots. Number of germinated seeds was recorded daily after sowing of seeds up to 50 days. A seed was considered germinated when visible protrusions of plumule observed. After 50 days seedlings were harvested. Root and shoot length, fresh and dry weight were recorded for each species and each treatment. The germination percentage was determined by counting the number of germinated seeds every day. Root and shoot dry weights were recorded after oven drying at 60oC for 48 hours.
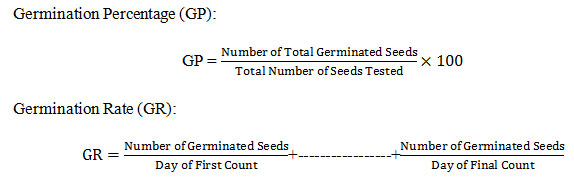
After final count germination percentage (GP) and germination rate (GR) was calculated by the following formulae19–
The shoots and roots were separated and the fresh weights were measured; after being oven dried at 60º C for 24 hours, the dry weights were taken immediately. According to each salt stress treatment, the fresh and dry weights in reference to control were calculated in percent, by using following equation:
3 (a): Fresh Weight (FW) Percentage Reduction
FWPR%= 100 [1: (Fresh Weight Salt Stress/Fresh Weight Control)]
3 (b): Dry Weight (DW) Percentage Reduction
DWPR%= 100 [1: (Dry Weight Salt Stress/Dry Weight Control)]
4: Relative Water Content (RWC):
The water content respective to the fresh weight was calculated as described by Sumithra20–
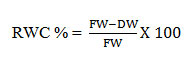
Seed Vigor Index (SVI):
This index was determined21–
![]()
6: Salt Tolerance Index (STI):
It is quantified by the ratio, respectively to the controlled, of the total dry weight in salt stress, (in percent), and calculated by the following equation:

Response Breadth (RB):
It was determined using following formula:
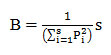
Where B is the niche breadth; Pi is the proportional response of species at ith treatment (water/salt stress level) and S is the number of size class.
8: Response Index (RI):
The Response Index (RI) was calculated as per the formula for the magnitude of inhibition versus stimulation by imposed stress on seed germination and seedling growth using following formula22:
When germination of treatments (T) is lower than the control (C):
RI = (T/C) – 1
When germination of treatments (T) is higher than the control (C):
RI = 1 – (C/T).
If RI > 0 Treatment stimulated germination
If RI = 0 No effect
If RI < 0 Treatment inhibited germination
Root: Shoot Ratio (R: S):

Leaf Weight Ratio (LWR):

Data were analysed statistically by using the software SPSS (version 16.0).
Results and Discussion
Analysis of variance showed significant effect on germination and seedling growth due to species as well as salt stress levels (Table1).
Table 1: Variance analysis (ANOVA) for traits investigated for the four species of Vigna in response to salinity stress.
|
Mean square |
|||||
| Parameters |
Df |
SL |
RL |
GP |
GR |
| Species |
3 |
15.55NS |
33.22* |
6982.30* |
0.011* |
| Salt Stress levels |
2 |
42.722* |
14.74* |
3497.72* |
0.009* |
* Significant at 5% level; Df: degree of freedom, NS: not significant, SL: Shoot length, RL: Root length,
GP: Germination percentage, GR: Germination rate.
Effect on Germination Percentage
In each species, salinity stress significantly affected germination percentage and germination decreased with increase in salinity level (fig. 1a). Maximum germination percentage (49.99%) was recorded in the control while the lowest germination (6.66%) was recorded for 100 mM salinity level. At control, maximum germination was observed in V. angularis (49.99%) and the minimum germination percentage was observed in V. aconitifolia (6.66%) while at high (100mM) salinity level, maximum germination (19.99%) was recorded for V. mungo and minimum (6.66%) for V. aconitifolia. Germination percentage showed a inverse relationship with salt stress levels (fig. 1a). The sensitivity index showed significant difference between the species in response to salt stress. The osmotic effect due to salinity was the main inhibitory factor that reduced germination.23 Salinity is one of the most important factors limiting plant growth and delaying seed germination as well as final germination percentage.24 The final germination percentage of the seeds treated with high salt concentration was much lowers than that of the control seeds, indicating that exposure to high concentration of NaCl strongly affected germination. Increasing concentration of NaCl probably caused the decrease in water potential gradient between the seeds and their surrounding media.25 According to the correlation coefficient, germination percentage was found to have a positive correlation with other measured parameters (Table 2).
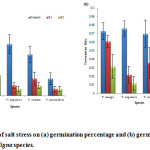 |
Figure 1: Effect of salt stress on (a) germination percentage and (b) germination rate of selected Vigna species. |
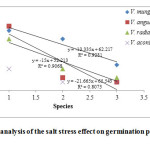 |
Figure 2: Regression analysis of the salt stress effect on germination percentage of Vigna species. Click here to View figure |
Table 2: Correlation matrix for analysed variables.
| SL | RL | GR | GP | SD | SVI | R : S | |
| SL | 1 | 0.9058 | 0.6827 | 0.8172 | 0.7859 | 0.9618 | 0.5251 |
| RL | 1 | 0.8118 | 0.9280 | 0.9030 | 0.8307 | 0.2552 | |
| GR | 1 | 0.9682 | 0.9823 | 0.7402 | 0.0538 | ||
| GP | 1 | 0.9979 | 0.8296 | 0.1325 | |||
| SD | 1 | 0.8106 | 0.1093 | ||||
| SVI | 1 | 0.4790 | |||||
| R : S | 1 |
SL: Shoot length (cm), RL: Root length (cm), GR: Germination rate, GP: Germination percentage, SD: Seedling dry weight (g), SVI –Seed vigour Index, R: S: Root: Shoot ratio.
Effect on Germination Rate
The highest germination rate was observed in control (0.076) and decreased as the salinity level increased (fig. 1b) which is in conformity to Kaya.26 Among species, V. angularis (0.012) showed lowest germination rate at all salinity levels as compared to other three species.
Effect on Initiation and Completion of Germination
The lowest emergence time taken by V. radiata was 9 days while rest of the three species took maximum 15 days for initiation (I) in controlled conditions. It has been reported that salinity delays germination27 and at high salinity levels seed germination features were deteriorated. The lowest completion time
(9 days) was taken by V. angularis while V. mungo (18 days) and V. aconitifolia (21 days) took more time to complete germination (Table 3).
Table 3: Effect of salinity stress on initiation and completion of seed germination of four Vigna species.
|
Species |
||||||||
| Salt stress |
V. mungo |
V. angularis |
V. radiata |
V. aconitifolia |
||||
|
levels |
I |
C |
I |
C |
I |
C |
I |
C |
| Control | 09.0±0.0 | 17.5±3.5 | 09.0±1.0 | 16.0±2.0 | 08.0±1.0 | 12.5±0.5 | 15.0±1.0 | 16.5±1.5 |
| 50mM | 09.5±0.5 | 16.5±1.5 | 08.5±0.5 | 09.0±1.0 | 08.0±1.0 | 09.5±0.5 | 10.0±0.0 | 11.0±0.0 |
| 100mM | 14.5±0.5 | 15.0±0.0 | 15.0±0.0 | 15.0±0.0 | 12.0±0.0 | 15.0±0.0 | 21.0±0.0 | 21.0±0.0 |
Effect on Seedling Length
In the present experiment, each species showed decline in root length with increasing salinity level. Among species, V. mungo (4.32 cm) showed maximum root length as compared to other three species (fig. 4a). The gradual decrease in root length with the increase in salinity as observed might be due to inhibitory effect of NaCl salt to root growth compared to that of shoot growth.28 Similarly, shoot length was decreased with increasing salinity stress. V. mungo (3.95 cm) produced maximum shoot length as a compared to V. angularis (2.34 cm), and V. radiata (2.45 cm) (fig. 3b). Shoot length was more suppressed than root by salinity at each salinity level. Plant height decreased with increasing salinity stress and V. radiata showed maximum susceptibility to salinity stress.
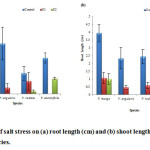 |
Figure 3: Effect of salt stress on (a) root length (cm) and (b) shoot length (cm) of selected Vigna species. |
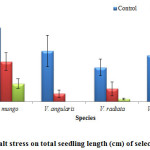 |
Figure 4: Effect of salt stress on total seedling length (cm) of selected Vigna species.
|
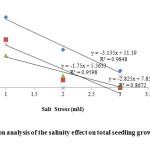 |
Figure 5: Regression analysis of the salinity effect on total seedling growth (cm) of Vigna species. |
Table 4: Means (± standard error) comparison of species, salt stress level and their interaction on the studied traits.
| Species |
SL |
RL |
GR |
GP |
SD |
SVI |
R : S |
|||
| V. mungo | 2.48±0.34 | 2.87±0.39 | 0.061±0.01 | 45.55±6.08 | 0.70±0.11 | 2.26±1.03 | 0.23±0.12 | |||
| V. angularis | 1.02±0.34 | 1.28±0.46 | 0.036±0.01 | 22.98±5.51 | 0.39±0.22 | 0.21±0.12 | 0.05±0.01 | |||
| V. radiata | 1.04±0.42 | 0.75±0.26 | 0.038±0.01 | 21.50±5.68 | 0.38±0.22 | 0.65±0.26 | 0.09±0.03 | |||
| V. aconitifolia | 1.05±0.52 | 0.56±0.26 | 0.015±0.01 | 8.88±3.16 | 0.18±0.04 | 0.30±0.12 | 0.19±0.05 | |||
| Treatment (Salt stress) | ||||||||||
| 0 mM | 2.95±0.61 | 2.37±0.56 | 0.06±0.007 | 38.33±6.65 | 0.021±0.001 | 3.15±0.79 | 0.12±0.04 | |||
| 50mM | 0.83±0.31 | 1.18±0.44 | 0.05±0.007 | 25.83±7.84 | 0.005±0.002 | 1.36±1.02 | 0.33±0.21 | |||
| 100mM | 0.41±0.20 | 0.54±0.27 | 0.015±0.006 | 10.04±5.54 | 0.004±0.001 | 0.21±0.08 | 0.17±0.09 | |||
SL: Shoot length (cm), RL: Root length (cm), GR: Germination rate, GP: Germination percentage, SD: Seedling dry weight (g), SVI –Seed vigour Index (%), R: S: Root: Shoot ratio.
Effect on Seedling Weight
V. angularis (0.21 g) has the maximum fresh weight (fig. 6a) as well as dry weight (0.03 g) (fig. 6b). The least affected species was V. aconitifolia with the continuous decrease in both fresh and dry weight with increasing stress levels (fig. 6a). Shoot length, root length and dry weight decreased with increasing salt stress in transgenic rice.29
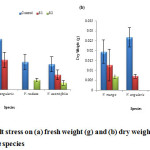 |
Figure 6: Effect of salt stress on (a) fresh weight (g) and (b) dry weight (g) of seedling of selected Vigna species. |
Effect on Relative Water Content
Relative water content was greatly influenced by salinity level. Among the species, V. mungo (17.86%) was most susceptible to salinity in terms of RWC and it showed lowest RWC at high salinity level (Table 5) while V. radiata showed better performance under the salinity stress. The water content was significantly reduced by the increase in NaCl concentration.30 Reduced water contents with increased salt stress was reported in Trigonella,31 while enhanced root moisture contents with increasing salinity levels was reported in Medicago polymorpha.32
Effect on Salt Tolerance Index
Salt tolerance index decreased with increasing salinity stress. In the present experiment, V. mungo (46.45%) showed the highest STI at 50 mM salt stress level while V. radiata showed the lowest STI (20.8%) at 100 mM salt stress level (Table 5). It has also been reported that salinity suppresses the uptake of essential nutrients like P and K which could adversely affect seedlings growth and vigor.33
Effect on Seed Vigor Index
Seed vigor index decreased with increasing salinity level indicating that salt concentration caused harmful effects on seeds.34 In the present experiment (Table 5), the maximum seed vigor index (4.40) was recorded at control for V. mungo and the lowest at 50 mM for V. angularis (0.41). Seedling vigor index of maize was also significantly affected under different salt stresses.35 It was reported that under stress conditions there is a decrease in water uptake both during imbibitions and seedling establishment and in the case of salt stress, this can be followed by uptake of ions.36 Seed vigour index had positive and significant correlation with salt stress levels.
Table 5: Effect of Salt Stress on different growth parameters of selected Vigna species.
|
Species / Salt stress levels |
FWPR (%) |
DWPR (%) |
RWC (%) |
SVI |
STI (%) |
|
50 mM |
|||||
| V. mungo |
92.17 |
53.53 |
17.86 |
04.40 |
46.45 |
| V. angularis |
97.73 |
77.94 |
47.31 |
00.41 |
23.54 |
| V. radiata |
– |
– |
– |
– |
|
| V. conitifolia |
– |
– |
– |
– |
– |
|
100 mM |
|||||
| V. mungo |
91.04 |
96.87 |
54.12 |
00.84 |
25.50 |
| V. angularis |
– |
– |
– |
– |
– |
| V. radiata |
98.24 |
79.19 |
29.47 |
00.64 |
20.80 |
| V. conitifolia |
07.55 |
70.76 |
78.04 |
01.23 |
29.23 |
FWPR: Fresh weight percent reduction, DWPR – Dry weight percent reduction, RWC: Relative water content, SVI: Seed vigour index, STI: Stress tolerance index.
Response Breadth (RB)
In the present study, V. mungo exhibited wider (0.88) response breadth as compared to other three species. In terms of response breadth species can be ranked as V. mungo>V. radiata>V. aconitifolia> V. angularis (fig. 7). Since V. angularis tend to show poor germination towards higher salt stress levels, it showed a narrower response breadth.
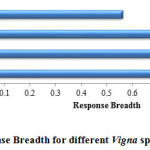 |
Figure 7: Response Breadth for different Vigna spp. under salt stress. |
Response Index (RI)
The Response Index (RI) was mostly negative with different level of salinity stress in all species for every variables (Table 6) indicating that germination and growth was negatively affected by salinity stress in each species.
Table 6: Response Index (RI) for seed germination, root length, shoot length and total seedling dry weight in different species of Vigna due to salt stress.
| Species |
Seed germination |
Root length |
Shoot length |
Total seedling dry weight |
||||
| 50 mM |
100mM |
50 mM |
100mM |
50 mM |
100mM |
50 mM |
100mM |
|
| V. angularis |
-0.78 |
-0.36 |
-0.69 |
– |
-0.35 |
– |
-0.83 |
– |
| V. mungo |
-0.56 |
-0.50 |
-0.16 |
-0.54 |
-0.54 |
-0.57 |
-0.28 |
-0.71 |
| V. radiata |
-0.50 |
-0.20 |
-0.23 |
-0.04 |
-0.33 |
-0.38 |
-0.61 |
-0.91 |
| V. aconitifolia |
-0.50 |
-0.25 |
– |
-0.30 |
– |
-0.40 |
– |
-0.79 |
Root : Shoot Ratio
Salt stress levels did not have any linear relationship with root: shoot ratio. Maximum root: shoot ratio was measured in V. aconitifolia (0.39) at 100mM salt stress level, while minimum root: shoot ratio was measured in V. radiata (0.02) (fig. 8a).
Leaf Weight Ratio
In the present study, leaf weight ratio showed variability with respect to salt stress levels. Maximum ratio was observed in V. angularis (0.81) at S1 (50 mM) salt stress level, while minimum was observed in V. aconitifolia (0.06) at S2 salt stress level (fig. 8b).
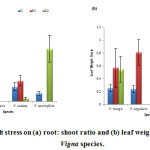 |
Figure 8: Effect of salt stress on (a) root: shoot ratio and (b) leaf weight ratio of selected Vigna species. |
Conclusions
In the present study, Vigna species were treated against salt stress at germination and early seedling growth stage and consequences unfolded vital information about their tolerance ability. Salinity exerts significant impact on every branch of growth parameters. Outcomes from the above study could be helpful in understanding the plant’s nature against different levels of salt stress and that could be economically exploited by various able agencies.
Acknowledgements
Financial support from ICSSR, New Delhi is gratefully acknowledged. We are thankful to the Head, Department of Botany for providing necessary facilities.
References
- Bargali, K and Bargali, S.S. Germination capacity of seeds of leguminous plants under water deficit conditions: Implication for restoration of degraded lands in Kumaun Himalaya. Trop. Eco. 57(3): 445-453 (2016).
- Bartels, D. and Sunkar, R. Water and salt tolerance in plants. Crit. Rev. Plant. Sci. 24: 23–58 (2005).
CrossRef - Pitman, M. and Lauchli, G. A. Global impact of salinity and agricultural ecosystems. In: Lauchli A, Luttge U (eds) Salinity: environment – plants – molecules. Kluwer, Dordrecht. 3-20 (2002)
- Garg, N. and Manchanda, G. Salinity and its effects on the functional biology of legumes. Acta Physiologiae Plantarum. 30: 595–618 (2008).
CrossRef - Vibhuti, Shahi, C. Bargali, K. and Bargali, S.S. Seed germination and seedling growth parameters of rice (Oryza sativa L.) varieties as affected by salt and water stress. Indian J. Agric. Sci. 85(1): 102-108 (2015a).
- Munns, R. Comparative physiology of salt and water stress. Plant Cell. Envir. 25: 239-250 (2002).
CrossRef - Yadav, S. S. McNeil, D.L. Redden, R. and Patil, S.A. Climate change and management of cool season grain legume crops. Springer, Dordrecht; Heidelberg, 460 (2010).
CrossRef - Heikal, M. M. Shaddad, M. A. and Ahmed, A. M. Effect of water stress and gibberellic acid on germination of flax, sesame and onion seed. Biol. Plant. 24: 124-129 (1981).
CrossRef - Nedjimi, B. and Daoud, Y. Effect of Na2SO4 on the growth, water relations, proline, total soluble sugars and ion content of Atriplex halimus subsp. schweinfurthii through in vitro culture. Ann. Biol. 28: 35-43 (2006).
- Goumi, Y. E. Fakiri, M. Lamsaouri, O. and Benchekroun, M. Salt stress effect on seed germination and some physiological traits in three Moroccan barley (Hordeum vulgare L.) cultivars. Ann. Biol. Res. 2(4): 490-497 (2011).
- Uhvits, R. Effect of osmotic pressure on water absorption and germination of alfafa seeds. American J. Bot. 33: 278-285 (1946).
CrossRef - Prisco, J.T. and O’leary, J.W. Osmotic and “toxic” effects of salinity on germination of Phaseolus vulgaris L. seeds. Turrialba. 20:177-184 (1970).
- Yokoi, S. Quintero, F. J. Cubero, B. Ruiz, M.T. Bressan, R. A. Hasegawa, P. M. and Pardo, J. M. Differential expression and function of Arabidopsis thaliana NHX Na+/H+ antiporters in the salt stress response. Plant J. 30: 529–539 (2002).
CrossRef - Rahdari, P. and Hoseini, S. M. Salinity Stress: A Review: Tech. J. of Eng. App. Sci. 1(3): 63-66 (2011).
- Shahi, C. Vibhuti, Bargali, K. and Bargali, S. S. Influence of seed size and salt stress on seed germination and seedling growth of wheat (Triticum aestivum L.). Indian J. Agric. Sci. 85(9):1134-1137 (2015).
- Pande, P.C., Vibhuti, Awasthi, P., Bargali, K. and Bargali, S.S. Agro-Biodiversity of Kumaun Himalaya, India: A Review. Curr. Agri. Res. J. 4 (1): 16-34 (2016).
CrossRef - Lluch, C. Tejera, N. Herrera-Cervera, J. A. Lopez, M. Barranco-Gresa J. R. Palma, F. J. Gozalvez, M. Iribarne, C. Moreno, E. and Ocana, A. Saline stress tolerance in legumes. Lotus Newslett. 37(2): 76-77 (2007).
- Raun, S. Xue, Q. and Thlkowska, K. Effect of seed priming on germination and health of rice (Oryza sativa L) seeds. Seed Sci. and Technol. 30: 451–458 (2002).
- Sumithra, K. Jutur, P. P. Carmel, B. D. and Reddy, A. R. Salinity induced changes in two cultivars of Vigna radiata: responses of antioxidative and proline metabolism. Plant Grow. Regul. 50: 11–22 (2006).
CrossRef - Vibhuti, Shahi, C. Bargali, K. and Bargali, S.S. Assessment of salt stress tolerance in three varieties of rice (Oryza sativa L.). J. Prog. Agric. 6(1): 50-56 (2015b).
- Williamson, G. B. and Richardson, D. R. Bioassay for allelopathy: Measuring treatment responses within dependent controls. J. Chem. Eco. 14: 181-187 (1988).
CrossRef - Akbar, M. and Ponnamperuma, F. M. Saline soils of South and Southeast Asia as potential rice land. In rice research strategies for the future. IRRI. 265-281 (1982).
- Rahman, M. S. Miyake, H. and Taheoka, Y. Effect of sodium chloride salinity on seed germination and early seedling growth of rice (Oryza sativa L.). Pakistan J. Biol Sci. 4(3): 351-355 (2001).
CrossRef - Bradford, J. K. Water relation in seed germination In: Kigel J and Galili G (eds) Seed development and germination. Marcel Dekker Inc. New York (1995).
- Kaya, M. Kaya, G. Kaya, M. D. Atak, M. Saglam, S. Khawar, K. M. and Cifti, C.Y. Interaction between seed size and NaCl on germination and early seedling growth of some Turkish Cultivars of Chick pea (Cicer arientinum L.). J. Zhejiang University Sci. B: Springer. 371-377 (2008)
- Hakim, M. A. Juraimi, A.S. Begum, M. Hanafi, M. M. Mohd, R. and Selamat, A. Effect of salt stress on germination and early seedling growth of rice (Oryza sativa L.). African J. Biot. 9(13): 911-1918 (2010).
- Rahman, M. S. Miyake, H. and Taheoka, Y. Effect of sodium chloride salinity on seed germination and early seedling growth of rice (Oryza sativa L.). Pakistan J. Biol. Sci. 4(3): 351-355 (2001).
CrossRef - Jamil, M. and Rha, E. S. Response of transgenic rice at germination and early seedling growth under salt stress. Pakistan J. Biol. Sci. 10:4303-4306 (2007).
CrossRef - Munns, R. James, R. A. and Lauchi A. Approaches to increasing the salt tolerance of heat and other cereals. J. Exp. Bot. 57: 1025-1043 (2006).
CrossRef - Shadded, M. A. and Zaidan, M. A. Effect of NaCl salinity on the rate of germination, seedling growth and some metabolic changes in Raphanus sativa L. and Trigonella foenum-graecum L. Beitrage Zur Tropischen Landwirtschaft Und Veterinarmedizin, 27(2): 187-194 (1989).
- Ibrar, M. and Hussain, F. The effect of salinity on the growth of Medicago polymorpha L. J. Sci. & Technol. Peshawar. 27: 35-38 (2003).
- Nasim, M. Qureshi, R. Aziz, T. Saqib, M. Nawaz, S. Sahi, S.T. and Pervaiz, S. Growth and ionic composition of salt stressed Eucalyptus camaldulensis and Eucalyptus teretcornis. Pakistan J. Bot. 40: 799-805 (2008).
- Cokkizgin, A. Salanity stress in common bean (Phaseolus vulgaris L.) seed germination. Notulae Botanicae Horti Agrobotanici. 40(1): 177-182 (2012).
- Janmohammadi, M. Dezfuli, P. M. and Sharifzadeh, F. Seed invigoration techniques to improve germination and early growth of inbred line of maize under salinity and drought stress. General Applied Plant. Physiol. 34: 215-226 (2008).
- Prisco, J. T. and Vieira, G. H. F. Effects of NaCl salinity on nitrogenous compounds and proteases during germination of Ligrra sinensis seeds. Physiol. Plant. 36: 317:320 (1976).
