Introduction
The Western Ghat of Southern India is recognized for its rich floral diversity of epiphytic and terrestrial orchids1. A major portion of the tropical Western Ghat passes through Kerala, which lies in the Southern region of India. Orchids are widely cultivated in this region due to their favorable climatic conditions and strong market demand. There are 310 recorded species of orchids in the Western Ghats, of which 123 are endemic to Kerala2. Orchids make the first choice of florists, gardeners, landscape artists, and in office spaces. The ecology, molecular biology, and breeding aspects of orchids have been extensively studied and advancement in new areas such as in-vitro flower initiation and pharmacological aspects are also underway3. The Western Ghats are facing severe losses to its orchid diversity due to habitat destruction, illegal collection from the wild, and other purposes such as cultural, food, and medicinal uses4. There is a great demand for imported hybrid orchids in southern India, and there is a well-established import system to cater to this demand. The ever-increasing demand for imported plants has increased the risk of the introduction of new invasive pests from foreign lands. To regulate the import, strict phytosanitary measures have been laid out by the Indian Government through The Plant Quarantine (Regulation of Import into India) Order (PQ order), 2003.
Strict quarantine regulations and quarantine legislation as per the PQ order 2003 of the Government of India are imposed on the import of orchids. According to5, two important orchid viruses Cymbidium mosaic virus (CymMV) and Odontoglossum ringspot virus (ORSV) viruses were detected in both native and imported orchids of India. These viruses had detrimental effects on the growth and development of orchids. Both were observed to be growth debilitating with high stability and high rate of spread6. A Survey for disease incidence in 36 different genera of orchids was conducted by 5, in different conservatories in Minnesota, USA. In the survey, the orchids were found to be infected by CymMV, ORSV, and OFV in varying degrees. In India, natural infection of orchid viruses has been detected across different genera of orchids viz; Bulbophyllum, Cattleya, Coelogyne, Cymbidium, Dendrobium, Epidendrum, Oncidium, Paphiopedilum, Phalaenopsis, Rhynchostylis, Vanda and Vanilla7,8. Virus-infected orchids showed smaller-sized, poor quality, necrotic flowers with blighted leaves, and fetching lesser prices in the market9. Both CymMV and ORSV were found to be transmissible through horticultural tools contaminated with virus-infected plant sap10. These viruses can pass undetected as asymptomatic plants, through regular quarantine checks and procedures. The plants showing latent infection may express the disease symptoms during later growth or reproductive stages11.
Nowadays high-throughput sequencing techniques are used to conduct surveys for virus infection followed by confirmation by genome sequencing, and phylogenetic analyses. In the study conducted by12, an uncharacterized virus Pterostylis blotch virus (PtBV), that infects greenhood orchids (Pterostylidinae) in the regions of New South Wales and Australian Capital Territory, Australia was elucidated. It was concluded from their study that this newly described virus was introduced from a foreign land with the possibility of an aphid vector. Similarly, Orchid fleck virus (OFV) was reported in regions of Mexico for the first time through advanced molecular detection techniques13. In India, a survey was conducted by14 in Sikkim and Darjeeling hills of North-Eastern India and observed the natural incidence of CymMV, Calanthe mild mosaic virus (CalMMV), Odontoglossum ringspot virus (ORSV), and Groundnut bud necrosis virus (GBNV) viruses on different genera of orchids using the particle morphology and DAC-ELISA with specific antibodies. In Meghalaya, among 84 leaf samples collected from orchid growers and nurseries, over 45% the incidence of both CymMV and ORSV were detected using multiplex RT-PCR15. There is also a great possibility of the presence of naturally infecting unknown viruses in these orchid hotspots of the Northeast and Western Ghats of India. The aim of the present study was to assess the incidence of orchid viruses in plant nurseries as well as in the Ghat region of Kerala. The study also aims at finding out the presence of important viruses diseases in wild orchids as well as the transmissibility of virus infection from cultivated orchids to the wild orchids.
Materials and Methods
Survey for disease incidence and Symptomatology
A survey was conducted during the years 2015-2020 for symptoms of viral disease incidence in native orchids in the Ghat region of Thiruvananthapuram, Idukki, Kottayam, Thrissur, Kozhikode, Wayanad, and Kasargod districts of Kerala (Image 1). Native Dendrobium orchids were collected from trees and rubber, areca, coffee, and cocoa plantations in the Ghats adjacent to reserved forests. The species-level identification of the collected native orchids was carried out with the help of taxonomists at M.S. Swaminathan Research Foundation, Wayanad, Kerala.
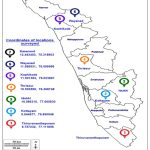 |
Image 1: Locations of survey and collection of native orchids from the Western Ghat region in seven districts of Kerala. |
Source: https://d-maps.com/carte.php?num_car=31783&lang=en
A similar survey for disease incidence in cultivated orchids was conducted by major orchid nurseries and growers in Thiruvananthapuram, Kottayam, Alapuzha, Ernakulam, and Thrissur districts of Kerala (Image 2). The survey was conducted among the growers who were involved in the import of orchids and those who maintained a post-entry quarantine (PEQ) facility. The imported live plants as per PQ Order 2003, should be maintained in the PEQ facility for a period before they are released. After the observation period on receipt of the release order for plants from the designated inspection authority, the plants are often shifted to greenhouses before they are sold to customers or retailers. The plants that develop symptoms of disease or pests during the observation period will be removed and destroyed during the inspection so that the chances of diseases escaping to nature are less. But virus diseases remain asymptomatic during the initial PEQ phase and are often expressed in the open field or greenhouse conditions. In this present study, a survey for virus disease was conducted in plants after the PEQ phase. Random samples (n=100), were selected from every location during the survey. The samples were selected from a mix of Dendrobium orchids containing different varieties. The overall disease incidence in orchids was calculated and expressed as a percentage. Percent disease incidence was calculated as per the formula16
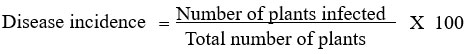
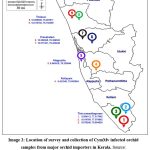 |
Image 2: Location of survey and collection of CymMv infected orchid samples from major orchid importers in Kerala. |
Source: https://d-maps.com/carte.php?num_car=31783&lang=en
Maintenance of virus inoculum and transmissibility study
The native varieties and cultivated orchids showing symptoms of viral diseases were collected from the locations and the plants were protected and maintained in an insect-proof glasshouse in the College of Agriculture, Vellayani, Thiruvananthapuram for further studies. The leaves exhibiting typical symptoms of virus disease such as mosaic and ring spots were used for mechanical transmission to assay host plants such as C. amaranticolor and D. stramonium to produce local lesions. (Image 3)
 |
Image 3: CymMV inoculated assay host plant showing local lesions. a) D. stramonium leaf at 40 dpi showing large blotches and yellow spots. b) C. amaranticolor leaf at 30 dpi showing necrotic of local lesions and overall withering.Click here to view Image |
Virus-infected orchid plants maintained at the greenhouse were used as the source of the virus. A homogenate of the infected leaf was prepared by grinding one gram leaf in 1.5 ml of 0.1 M phosphate buffer (pH 7.0) in pre-chilled mortar and pestle. The assay host plant (C. amaranticolor) was raised inside the insect-proof greenhouse and inoculation was carried out at eight leaved stages of the seedling. For mechanical inoculation, the leaves of assay host plants were uniformly dusted with 600 mesh size carborundum powder and gently rubbed the leaves using the forefinger dipped in leaf homogenate. The inoculum was left to retain on the leaf for about three min and then it was washed away with distilled water from a squeeze bottle. The plants were then maintained in the greenhouse to observe symptom development. The sap transmission of CymMV was done on fully opened young leaves of healthy plants to study the infectivity of both viruses. Observations in leaves, stems, side shoots, roots, and flowers were carried out at intervals at specific days post-inoculation (5, 20, 60, 90, and 120 dpi).
Detection and cloning of CymMV in cultivated orchids.
The presence of orchid viruses was detected by using DAC-ELISA. Leaf tissues from over 2000 samples that were suspected of the presence of the virus or showing characteristic symptoms of viral infection from seven different species of orchids viz.; Dendrobium sp., Cattleya sp., Cymbidium, Epidendrum sp., Grammatophyllum sp., Mokara sp., and Vanda sp. were analyzed. Tender shoots, roots, and flowers were used for the detection of viruses. Two specific antibodies of CymMV and ORSV and two non-specific antibodies of Cucumber mosaic virus (CMV) and Potato virus Y (Potyvirus) that were available at the Department of Plant Pathology, College of Agriculture, Vellayani, Kerala were used. The procedure described by17, with minor modifications was followed for detection.
PCR-amplified coat protein gene of the CymMV strain isolated from cultivated Dendrobium orchids from the Thiruvananthapuram district was selected for cloning and constructing the phylogeny. Cloning and sequencing were carried out using the InsTAclone PCR cloning kit (Thermo Scientific) with slight modifications. The E. coli vector pUC18 was available at the Department of Biotechnology, College of Agriculture, Vellayani, Thiruvananthapuram, Kerala was used as a cloning vector. Competent cells of E. coli (DH5α) were prepared by treatment with Calcium chloride (CaCl2). The pUC18 vectors carrying the CP gene of CymMV were used for transformation into competent E. coli (DH5α) maintained in LB agar. The transformed cell suspension was plated (150 μl per 9 cm Petri plate) in LB agar supplemented with Ampicillin (Amp), Isopropyl β- d-1-thiogalactopyranoside (IPTG), and 5-bromo-4-chloro-3-indolyl-β-D-glucopyranosides (X-Gal) and incubated overnight at 37oC. The transformed cells were screened based on the blue-white colony selection method. The colonies of E. coli cells that were successfully transformed were selected and lysed by the alkali lysis method explained by18. The cell lysate thus obtained was used for PCR using specifically designed primers for CymMV CP. For PCR, 2 μl of the cell lysate was taken using a micropipette (Tarsons), and the PCR was run for 25 cycles at 94oC/2 mins (initial denaturation) followed by 94oC/30 sec (denaturation), 72oC/1 min (annealing), and 72oC/ 5 mins (extension). The PCR products were checked for confirmation of amplicon using agarose gel electrophoresis with a 100bp DNA ladder as reference. The amplicon was then sequenced using the CymMV CP-gene-specific primer. The sequence thus obtained was compared with similar sequences available in the NCBI database using Basic Local Alignment Search Tool (BLAST). The sequences showing the most similarity was aligned with CLUSTAL Omega, a multiple sequence alignment tool, and MEGA 11 was used to study and construct the phylogeny by the maximum-likelihood (ML) method using the 1,000 bootstrap replications.
Results
Survey for disease incidence and Symptomatology
Among orchids, Dendrobium, Phalaenopsis, Mokarra, Oncidium, and Cattleya were imported in large quantities. The number of firms involved in the plant importation to Sothern India especially to Kerala has seen a steady rise over the decade. As per the records from the office of the Designated inspection authority for imports of plants and planting materials of Kerala Agricultural University, before 2006 there were less than five firms who were involved in live plant importation. With the enactment of PQ Order 2003 and the trade liberalization policies of GoI, more firms started investing in the live plant import business. Even farmers and individual plant nursery owners have started to import live plants (Image 4).
The survey in five districts of Kerala on a total of 9500 plants. During this most of the samples tested positive for CymMV and very few samples tested positive for ORSV. The minimum disease incidence of 5% was recorded in the Alappuzha district and the highest incidence (100%) was recorded in the Thiruvananthapuram district (Table 1). The most common symptoms were slight chlorotic patterns, partly or fully covering the leaf surface. Other symptoms were irregular chlorotic patches on leaves, which may appear similar to that nutrient deficiencies, physiological disorders, or mite infestation (Image 5). Plants appeared generally weak with fewer leaves. In older leaves of Dendrobium plants, symptoms became severe and appeared as general yellowing with sunken patches on the leaf surface. Whereas in younger leaves, narrow streaks and spindle-like mosaic patterns were observed.
 |
Image 4: Imported Dendrobium plants maintained in a close arrangement in a post-entry quarantine (PEQ) facility. |
 |
Image 5: Dendrobium plants affected by Cymbidium mosaic virus (CymMV) in growers’ polyhouse showing irregular chlorotic patches on leaves. |
Floral parts were also affected; flower buds turned necrotic, reduction in the number and flower size of flowers per peduncle was observed during severe infection. Flower colour break, a typical symptom of CymMV was not observed in Dendrobium orchids anywhere but the cultivated variety of Cattleya sp. exhibited flower colour break symptoms (Image 6). Visible symptoms were not observed on the stem and the roots. Plants that were maintained in less than 50% shade had light-coloured leaves and therefore symptoms were not distinguishable. Whereas the plants maintained in greenhouses with more than 50% percent shade had darker-coloured leaves, thereby making mosaic symptoms more distinguishable.
 |
Image 6: Cattleya sp. (lavender flower variety) infected with CymMV showing colour-breaking symptoms on petals. |
Table 1: Locations of survey and incidence of CymMV/ ORSV in Dendrobium orchid growers and importers
| Sl. No | Location of survey (District wise) | Number of plants surveyed | Viruses detected | Virus disease incidence (%) |
| 1. | Thiruvananthapuram | 5500 | CymMV | 10-100 |
| 100 | ORSV | 30 | ||
| 2. | Alappuzha | 400 | CymMV | 5.0 – 40 |
| 3. | Kottayam | 1000 | CymMV | 10 – 60 |
| 100 | ORSV | 40 | ||
| 4. | Ernakulam | 1000 | CymMV | 20 |
| 1200 | CymMV | 50 – 75 | ||
| 5. | Thrissur | 200 | CymMV | 20 |
| 200 | CymMV | 5.0 |
Maintenance of virus inoculum and transmissibility study
The C. amaranticolor leaves were inoculated with CymMV-infected plant sap extracted from leaves of infected Dendrobium orchids (0.1M phosphate buffer pH 7.0). developed small blotchy lesions that could be distinguished from 15 days post-inoculation (dpi). The lesions gradually increased in size and progressed to necrosis at 30 dpi. The inoculated leaves fell off from the plant after 30 dpi. The leaves on control plants remained symptomless and intact at 30 dpi. ORSV-inoculated leaves did not produce any local lesions 30 dpi. In D. stramonium, CymMV was inoculated into the first fully developed leaf of a four-leaf stage plant. The inoculated leaves developed blotchy spots at 20 dpi. The spots turned into larger-sized lesions with yellow halo at 20 dpi and later the same leaves turned necrotic and fell off the plants at 40 dpi.
The imported planting material was observed as the primary infection source for CymMV and ORSV in plant nurseries during the survey. Most of the plant importation was from Southeast Asian countries like Thailand, Taiwan, Singapore, and Malaysia. The plants were maintained in close arrangement during the transit, as well as the PEQ period. The practice of disinfection of pruning tools and knives was not practiced by growers or in PEQ facilities. The plants were found to be vegetatively propagated through suckers and side shoots in different locations of Trivandrum and Alappuzha. In Ernakulam district, mother plants maintained in poly houses tested positive for the CymMV in DAC-ELISA.
Transmissibility of CymMV on native orchids
The CymMV was observed to be highly sap transmissible. Healthy orchids were infected with CymMV by sap transmission methods such as slash, piercing, and abrasion using carborundum. The systemic movement of CymMV and ORSV in Dendrobium upon artificial inoculation was found to be quickest in leaves. The movement of the virus from leaf tip to root tip was three months post-inoculation for CymMV and four months post-inoculation in ORSV. Orchids other than Dendrobium sp. were found to exhibit symptoms of CymMV during the survey. Cultivated varieties of Cattleya sp., Cymbidium sp. (Image 7), Epidendrum sp., Grammatophyllum sp. (Image 8), Mokara sp., and Oncidium sp. showed symptoms of CymMV in varying degrees. All the cultivated orchids took up the virus infection quickly and the symptoms were expressed within two months. Whereas many of the wild orchids did not take up the infection and the symptom expression were not as prominent as in the cultivated species (Images 9 & 10) (Table 2).
 |
Image 7: Cymbidium sp. infected by CymMV showing symptoms with necrotic patches. |
 |
Image 8: CymMV-infected Grammatophyllum sp. showing streak-like mosaic patterns on leaves. |
 |
Image 9: Native species of Dendrobium sp. from different parts of the Western Ghat of Kerala were tested for natural incidence of virus infection. (a D. haemoglossum, b. D. aqueum, c. D. nutans). |
 |
Image 10: Acantheppium bicolor which was artificially inoculated with CymMV showed mosaic symptoms along the interveinal region. |
Table 2: Survey for natural incidence of CymMV and the symptom development upon artificial inoculation in cultivated and wild orchids of Kerala.
| Sl. No. | Orchid varieties/species | Location of survey | Natural incidence of CymMV | Symptoms observed upon artificial inoculation of CymMV |
| 1. | Dendrobium haemoglossum | Wayanad | No natural infection | Yellow diffused discoloration in 40 dpi. |
| 2. | Dendrobium aqueum | Idukki, Wayanad | No natural infection | Yellow blotchy discoloration in 60 dpi. |
| 3. | Dendrobium nutans | Wayanad | No natural infection | A yellow diffused pattern developed after 24 dpi |
| 4. | Dendrobium ovatum | Wayanad, Idukki | No natural infection | Yellow diffused mosaic in 60 dpi. |
| 5. | Cattleya sp. | Kottayam, Thiruvananthapuram, Thrissur, | No natural infection | Diffused mosaic in 60 dpi upon mechanical inoculation |
| 6. | Grammatophyllum p. | Thiruvananthapuram | No natural infection | No symptom development even after 90 dpi |
| 7. | Oncidium sp. | Kottayam, Kozhikode, Thiruvananthapuram, Thrissur | No natural infection | Yellow discoloration and mosaic pattern in 30 dpi |
| 8. | Epidendrum sp. | Thiruvananthapuram, Thrissur | Natural infection observed in cultivated varieties | Diffused mosaic in naturally infected plants |
| 9. | Mokara sp. | Thiruvananthapuram | No natural infection | Diffused mosaic in natural infection |
| 10. | Cymbidium aliofolium | Kottayam, Idukki, Thiruvananthapuram, Thrissur, Wayanad | Natural infection observed in wild varieties | Diffused mosaic when mechanically inoculated and dark necrotic patches in naturally infected plants |
| 11. | Acanthophippium bicolor | Wayanad | No natural infection | Sparse interveinal yellow discoloration in 50 dpi |
| 12. | Vanda tessellata | Thiruvananthapuram, Kozhikode, Kasargod | No natural infection | Diffused mosaic when mechanically inoculated and dark necrotic patches |
| 13 | Acampe praernorsa | Wayanad | No natural infection | No symptom development even after 90 dpi |
| 14 | Arundina graminifolia | Wayanad | No natural infection | No symptom development even after 90 dpi |
| 15 | Cottonia peduncularis | Wayanad | No natural infection | No symptom development even after 90 dpi |
| 16. | Liparis viridiflora | Wayanad | No natural infection | No symptom development even after 90 dpi |
| 17. | Luisia zeylanica | Kozhikode, Wayanad | No natural infection | No symptom development even after 90 dpi |
| 18. | Pholidotta pallida | Thiruvananthapuram, Wayanad | No natural infection | No symptom development even after 90 dpi |
| 19. | Oberonia sp. | Wayanad | No natural infection | No symptom development even after 90 dpi |
Detection and cloning of CymMV in cultivated orchids
DAC-ELISA analysis on symptomatic samples of seven cultivated genera of orchids showed the presence of CymMV, ORSV, and mixed infection of both CymMV and ORSV. In CymMV-infected Dendrobium plants, the highest virus titre value was observed on fully opened new leaf tissue. Viral load was found to be the least in tender stems of the CymMV-infected plants. In samples showing symptoms of ORSV, the highest virus titer was observed on leaves showing characteristic ring spot symptoms. The lowest virus titer was observed in root tissue for ORSV-infected plants. In orchids with mixed infection of both CymMV and ORSV, the highest virus titer value was that of CymMV on fully opened symptomatic leaves.
Competent E. coli (DH5α) cells produced by CaCl2 treatment were maintained and stored in an ice box overnight. The cloned pUC18 vector was used for transforming into competent E. coli (DH5α) cells and immediately plated into LB agar supplemented with Amp, IPTG, and X-Gal. After 24 hr of incubation, plates containing transformed colonies of E. coli cells produced white colonies (10 – 15 numbers per Petri plate), no blue colonies were observed in plates carrying transformed cells. Colony PCR on transformed cells followed by agarose gel electrophoresis and the final sequenced CymMV CP gene was 642bp in length. This CP sequence showed 94% sequence similarity to the ‘Cymbidium mosaic virus coat protein gene, complete cds submitted from Taipei, Taiwan’ (Accession no. AY429021.1). The phylogeny constructed based on the NCBI-BLAST result showed a similar pattern based on sequence similarity and the query cover. In the phylogeny tree constructed, there were two groups of CymMV observed. The first group includes CymMV isolate from Thiruvananthapuram with CymMV strains from Brazil and South Korea and the second group comprises strains from Taiwan, India, China, and Singapore strains of CymMV (Image 11).
 |
Image 11: The phylogeny constructed based on the NCBI-BLAST result shows two major groups’ patterns based on sequence similarity and the query cover. |
Discussion
Orchids are popular ornamental flowers around the world for their beautiful flowers, wide range of colours, varieties, and ease of management. It can be easily grown indoors, as well as outdoors under partially shaded conditions. Orchids are grown for gardening, cutting flowers, and landscaping. Among many species of orchids, Dendrobium sp. is widely cultivated in India and Kerala and is the most preferred orchid species among growers for its flower quality, ease of management, and availability of a wide range of varieties/hybrids both indigenous and exotic. A majority of these plants are brought into the country from South-East Asian countries. Pathogens such as fungi, bacteria, and viruses affect orchid plants at different stages causing rots, wilts, and necrosis at different stages of plant growth. Orchids around the world are infected by more than 30 different viruses19. Among the different orchid viruses, CymMV and ORSV are ubiquitous throughout the orchid growing area in the world20.
In the survey conducted among the major orchid growers of Kerala, during 2015-2019, all the surveyed locations had a CymMV incidence of not less than five percent. The survey was conducted by21 Dendrobium plants maintained in orchid nurseries in different locations of Tamil Nadu recorded an incidence of the CymMV up to 22 – 58% in different locations. The disease incidence varied mostly according to the age of the plant, horticultural practices, and imported mother plants. Well maintained plants with adequate sunlight, nutrition and shade conditions were found to be apparently healthy with latent infection.
Surveys for disease incidence in orchids have been carried out in different parts of India such as the survey by7, where samples of Cymbidium sp. from various locations in Sikkim and Darjeeling were analyzed during the year 2007-08 and in different orchid nurseries in Sikkim and three nurseries from around the Darjeeling hill for the detection of orchid viruses. Mostly, CymMV and ORSV were found in mixed infections in many samples of native and hybrid Cymbidium sp. In a similar survey conducted in vanilla plantations of Kerala and Tamil Nadu by22, from sixty-five locations, the incidence of CymMV was commonly detected in vanilla plants. The survey was effective in determining the presence and extent of the spread of CymMV in vanilla plants, which was earlier unknown in this region. The survey helped to reveal the extent of spread and severity of the virus in vanilla plants for taking precautionary actions. Recent developments in rapid on-field detection of plant viruses have been demonstrated in orchid viruses by23. The triplex TaqMan quantitative real-time PCR technique allowed rapid detection of multiple orchid viruses using highly sensitive probes and specifically designed primers. The present study used DAC-ELISA technique with specific primers for detection of CymMV. Even though this was a time-consuming and destructive sampling was required, it was effective in determining the presence and the extent of the spread of CymMV in Dendrobium orchids in different parts of Kerala.
In our observation of imported Dendrobium, all the plants belonging to the same age were selected for scoring during the survey. In this way, it was easier to trace the origin, location of importation, and schedule of operations carried out on the plants during the PEQ period. In the survey, disease incidence of CymMV greater than 60% was recorded in multiple locations and the symptoms expressed in orchids varied with factors like light intensity, moisture, and temperature and with the age and nutrition of plants. Expression of symptoms also varied between different species of orchid. Mosaic, mottling, streaking, and depressions on leaf tissues were the most common symptoms observed in most of the orchid species. The first report of the presence of CymMV in Cymbidium orchids was made by24 and it was initially named Cymbidium black streak virus, due to the presence of black necrotic spotting on leaves. The characteristic necrotic spotting was observed in Cymbidium sp. plants during the present study but not in any other species of orchids.
Dendrobium plants were observed to appear chlorotic, weak with fewer leaves on plants. Floral parts were also found to be affected with common symptoms like flower buds turning necrotic, reduction in flower size, and lesser flowers per peduncle observed during severe viral infection. Flower colour break, a typical symptom of CymMV as described by25, was not observed in any dendrobium plants in the locations surveyed during the present study. The infection remained latent in plants and was carried over to the next plants through vegetative propagation and tissue culture.
The symptom production in Dendrobium leaves due to CymMV was found to be like that described by26, where leaves exhibited irregular mosaic, chlorosis, sunken patches, and petal colour break with necrosis on flowers. A study by25, described similar symptoms in CymMV, such as chlorotic or necrotic patches with sunken epidermis on orchid leaves. Similar symptoms were also observed on flowers. The flowers of CymMV-infected plants were observed to develop necrosis, petal deformation, and colour-breaking symptoms. The CymMV infection on orchid flowers resulted in smaller-sized flowers with poor quality9. Necrosis and flower disfigurement of flowers when combined with symptoms on foliage, resulted in great economic losses.
ORSV-infected plants under our observation showed varying symptoms which include ring spots, dark patches, necrotic spots, and yellowing as described in the first report of ORSV by24. The infected older leaves turn yellow and brittle and dropped off prematurely in about two months, severely infected plants had only fewer leaves on them. Newly infected plants showed physiological stress and severe symptom expression during the initial phase after virus inoculation and later remained asymptomatic. Few cases of mixed infection by both CymMV and ORSV were observed in Dendrobium plants during the survey. In nurseries, most plants were found to be asymptomatic due to proper management and good horticultural practices. Flowers were found to be fewer in number on the peduncle, unopened flower buds showed symptoms of pitting and necrosis and dropped off prematurely without blooming.
The symptoms reported by25, by ORSV infection in native cymbidium plants were in concurrence with the present study on Dendrobium orchids. ORSV caused symptoms such as streaks or striped mosaic, diamond mottling, or ring spots on leaves. Flowers of infected plants exhibited coloured ringspot and colour-breaking symptoms. In combined infections, the symptoms of both viruses were severely expressed. Many times, infected orchids asymptomatic and indistinguishable from healthy orchids and go undetected. Mixed infection of CymMV and ORSV in orchids had severe physiological damage to the host plant7. The infection severely affected normal physiological activity, especially flowering.
The virus-infected plants maintained in the greenhouse at the College of Agriculture, Vellayani, Thiruvananthapuram showed variation in symptoms during the period of study. C. amaranticolor and D. stramonium were selected for the maintenance of virus inoculum as it was described as better assay hosts for CymMV by27. In C. amaranticolor the blotchy lesions turned into necrotic during later stages and the leaves dropped off the plant. In D. stramonium, blotchy spots turned into larger-sized lesions with yellow halo and later the leaves turned necrotic and fell off the plants. We observed no systemic movements of CymMV in C. amaranticolor and D. stramonium. Maintenance of viral inoculum in assay host plants such as C. amaranticolor and D. stramonium was effectively carried out by7, and these plants were found to be better assay hosts than commonly used assay hosts like Phaseolus vulgaris, Nicotiana glutinosa, N. rustica, and Physalis floridana.
The sap inoculation of CymMV was used by28, for partially purified CymMV and TMV-O to be inoculated into Cattleya pseudobulbs by using carborundum powder, syringe, and superficial cuts made using a razor dipped in viral suspension for maintenance of inoculums and study of symptom expression. The purified viral suspension was inoculated into D. stramonium to get local necrosis-like symptoms. N. benthamiana plants were used to maintain CymMV and ORSV through local lesion assay for further purification of the virus and production of antisera29. In the present study, carborundum was effectively used for successful mechanical inoculation in both orchids as well as in assay host plants. Razor cuts were used only in the transfer of ORSV to healthy Dendrobium plantlets, where inoculation using carborundum was not found to be effective.
The assay hosts such as Chenopodium sp. were effective in determining strain variations or unknown viruses in orchids. C. quinoa was used as a test host plant to study an unknown virus, infecting Phalaenopsis plants (Phalaenopsis chlorotic spot virus isolate 7-2)30. The viral inoculums were initially established and maintained in C. quinoa and N. benthamiana plants for further morphological and molecular characterization. C. amaranticolor was used in the present study and CymMV could be successfully maintained in plants for over a month.
C. amaranticolor was the best assay host for the detection of CymMV than for ORSV. Nicotiana benthamiana was observed to be a good assay host for ORSV which is the most frequently used diagnostic plant for biological detection of CymMV. In the present study. C. amaranticolor inoculated with CymMV showed slow developing blotchy lesions on leaves that were large enough to cover most of the leaf surface. The lesions were localized, and no systemic infection was observed in the test host plant. The symptoms of CymMV infection on C. amaranticolor leaves were not observed as typical hypersensitive response and the symptoms slowly progressed to necrosis 7,26.
In D. stramonium, the leaves developed blotchy spots at 20 days post-inoculation upon inoculation with CymMV. C. amaranticolor produced definite local lesions unlike D. stramonium so it was used for screening the efficacy of antiviral principles in the present study7, carried out a similar study where mechanical inoculation from sap extracted from symptomatic leaves of native orchid plants collected from different parts of Sikkim. The inoculation of CymMV into young seedlings of D. stramonium exhibited small, pointed lesions on younger leaves which later turned to large necrotic lesions. Whereas C. amaranticolor developed slowly blotchy local lesions but no systemic movement was observed which is similar to the observations in the present study.
In a study by30, 29 different species of plants from nine different families were for the biological characterization of Phalaenopsis chlorotic spot virus isolate 7-2 (PhCSV isolate 7-2), from Taiwan. The virus was mechanically inoculated into the test plants using similar techniques and it was also back inoculated to Phalaenopsis seedlings to confirm the presence of PhCSV. The characteristic symptoms produced in the wild plant were found to be strongly similar to the replicated symptoms. This explains the effectiveness of using assay hosts for easy detection of mechanically transmissible plant viruses. The major viruses of orchids such as CymMV and ORSV are highly mechanically transmissible and hence detected using assay host plants.
The CP gene-carrying transformed colonies of E. coli cells produced white colonies in plates with no observed blue colonies. The degradation of X-gal in the LB agar plate may be the reason for no blue colonies observed during the culture. The final sequenced product of the CymMV CP gene was 642bp which showed 94% sequence similarity to ‘Cymbidium mosaic virus coat protein gene, complete cds submitted from Taipei, Taiwan’ (Accession no. AY429021.1) similar to that of the results obtained by22 in CymMV CP gene of virus isolates obtained from cultivated V. plainifolia plants. The V. plainifolia isolate gave a gene product of 672 nucleotides and it showed 98.9 percent sequence similarity to CymMV isolate from Singapore. The Potex virus genes are highly conserved, especially in the CP gene hence do not show much variability in these regions as suggested by 8,31, 32.
Specifically designed primers are generally used to clone the biologically active full-length sequences of CymMV and ORSV. Whereas the cDNA sequences obtained by RT-PCR were cloned into pBluescript KS+ vector at BamH1 and SmaI sites by three-piece ligation sequence instead of the single site as in the present study. The efficiency and infectivity of full-length clones were tested by infecting the clones with leaves of Nicotiana benthamiana. The viral clones were then subjected to Western blot and RT-PCR and confirmed by gene sequencing33. In a similar study34, with CymMV isolate from V. fragrans from the Hainan region of China, the PCR amplified complete CymMV cDNA (6224 nucleotides) products were cloned into pMD18-T vector and then transformed into E. coli (DH5α) cells. The colonies that had positive clones with target inserts were identified by using colony PCR technique. The Hainan isolate of CymMV from V. fragrans was found to share 96.61 percent sequence similarity to that of the Hawaiian isolate of CymMV (Accession no. EF125180).
Orchids belonging to other species were also found to carry infection of CymMV in nurseries during the survey. The infection of CymMV was found only in nurseries that propagated and maintained imported dendrobium plants. Native species of dendrobium i.e., D. haemoglossum, D. aqueum, and D. nutans did not carry natural infection of CymMV. These plants easily took up infection when artificially inoculated showing the possibility of spread of infection into native varieties. Orchids other than dendrobium viz., Cattleya sp., Grammatophyllum sp., Oncidium sp., Epidendrum sp., Mokara sp., and Cymbidium sp. were found to be infected in nurseries or easily took up the infection when mechanically inoculated. Whereas the study by7 where 100 different species of orchids were collected in a survey reported the occurrence of mixed infection of CymMV and ORSV in as many as 41 species of orchids. Among them, 17 different species of epiphytic and ground orchids tested positive for CymMV alone, suggesting that different species of orchids can be naturally infected or can easily take up the infection. The survey was carried out mainly in the nurseries of Darjeeling and Sikkim, showing the possibility of the spread of viruses through mechanical means. In a similar study6, the gardeners working in orchid nurseries were found to assist in the spread of viruses through gardening tools. Orchids such as Cymbidium sp., Odontoglossum sp., Oncidium sp., and Phalaenopsis sp. exhibited variations of symptoms of CymMV in their flowers and vegetative tissues.
Research works on the management of virus diseases of orchids using transgenics, root endophytes and antiviral compounds are underway to protect the commercial orchid industry worldwide. Resistant lines were developed using pathogen derived resistance and it was successfully demonstrated to confer dual resistance against CymMV and ORSV by35. The transgenic plants were reported to have 70-90 % lower virus replication in the protoplast when compared to the protoplasts of the control plants. Moreover, the viral load in these transgenic lines was very low due to low cell-to-cell as well as long-distance systemic movement in the test plants. The use of artificial micro RNAs (miRNAs) was also found to confer resistance to Nicotiana benthamiana test plants against orchid viruses. The miRNA technique can be replicated in orchids to confer resistance against multiple viruses during commercial cultivation36. Control of CymMV using root endophytes in combination with antiviral compounds was demonstrated in Dendrobium orchids by37. The techniques in reducing the virus inoculum at the origin or source from which the plants are imported will help to reduce the spread of orchid viruses. In the case of the present study, the major importers of orchid plants must be educated about the potential threat caused by these viruses and undertake adequate management measures.
Acknowledgement
The authors are grateful to orchid growers and importers of Kerala for their wholehearted support and for providing infrastructure for the study. We would also like to express our gratitude to the local farmers, MS Swaminathan Research Foundation, Wayanad, Kerala, and faculty members and scientists of the Department of Plant Pathology, College of Agriculture, Vellayani, Kerala for their support and timely help. We are deeply grateful for the research grant provided by Kerala Agricultural University (Approval code for the research: PPBM-01-00-06-2016 ACV(05)-KAU-PG) and the laboratory facilities by the Department of Plant Pathology, College of Agriculture, Vellayani, Kerala.
References
- Ajithkumar K, Pandiyarajan R. Aswini A. Conservation of orchids in the Western Ghats Region of Kerala, India. Acta Horticulturae. 2017; 57-62. 10.17660/ActaHortic.2017.1165.8.
CrossRef - Jayanthi J, Jalal J S. Endemic orchids of peninsular India: a review. Threatened Taxa. 2012; 4(15): 3415–3425.
CrossRef - Zhao D, Hu G, Chen Z, Shi Y, Zheng L, Tang A, Long C. Micropropagation and in vitro flowering of Dendrobium wangliangii: A critically endangered medicinal orchid. Medicinal Pl. Res. 2013; 7(28): 2098-2110.
CrossRef - De L, Medhi R. Diversity and Conservation of Rare and Endemic Orchids of Northeast India -A Review. Indian J. Hill Farming. 2019; 27(1): 138-153.
- Bratsch S A, Olszewski N, Lockhart B. Incidence of Cymbidium mosaic, Odontoglossum ringspot, and Orchid fleck virus in orchids in Minnesota and production of antibodies for use in ELISA to detect Orchid fleck virus. J. Plant Pathol.2021; 159: 543–554.
CrossRef - Koh K W, Lu H. Chan M. Virus resistance in orchids. Plant Sci. 2014; 228: 26–38.
CrossRef - Pant, R. P., Das, M., Pun, K. B., Ramachandran, P., Medhi, R. P. Occurrence of Cymbidium mosaic and Odontoglossum ringspot viruses in orchid germplasm of Sikkim and Darjeeling hills, their identification and diagnosis, Indian Phytopath. 2010; 63(3): 326-332.
- Sherpa A R, Bag T K, Hallan V, Zaidi A A. Incidence of Cymbidium mosaic virus (CymMV) in Sikkim. Indian Phytopath. 2007; 60 (1): 133-136.
- Seoh M L, Wong S M, Zhang L. Simultaneous TD/RT-PCR detection of Cymbidium mosaic potexvirus and Odontoglosum ringspot Tobamovirus with a single pair of primers. Virol. Methods. 1998; 72(2), 197-204
CrossRef - Moles M, Delatte H, Farreyro K, Grisoni M. Evidence that Cymbidium mosaic virus (CymMV) isolates divide into two subgroups based on nucleotide diversity of coat protein and replicase genes. Virol. 2007; 152: 705–715.
CrossRef - Safeer M M, Umamaheswaran. Occurrence of Cymbidium Mosaic Virus in Dendrobium Orchids in Kerala and Its Management through Meristem Culture. Indian J. Pure Appl. Biosci. 2021; 9(1): 267-275.
CrossRef - Chao H Y, Clements M A, Mackenzie A M, Dietzgen R G, Thomas J E, Geering A D W. Viruses Infecting Greenhood Orchids (Pterostylidinae) in Eastern Australia. Viruses. 2022; 14(2):365.
CrossRef - Otero-Colina G, Ramos-González P L, Chabi-Jesus C, Freitas-Astúa J, Tassi A D, Kitajima E W. First detection of orchid fleck virus in orchids in Mexico. Virus dis. 2021; 32(1), 167–172.
CrossRef - Rashmi R E, Pant R P, Basavaraj Y B, Baranwal V K, Jain R. Detection of Orchid Viruses and Molecular Characterization of Odontoglossum Ringspot Virus (ORSV) Isolates. The Indian J. Agrl. Sciences. 2021; 91 (8): 168–1173.
CrossRef - Nongsiang A, Das M C. Simultaneous detection of Odontoglossum ringspot virus (ORSV) and Cymbidium mosaic virus (CymMV) infecting orchids in Meghalaya, India by Multiplex RT-PCR, South African J. Bot. 2022; 151, 774-780. doi.org/10.1016/j.sajb.2022.10.036.
CrossRef - Pradhan S, Regmi T, Ranjit M, Pant B. Production of virus-free orchid Cymbidium aloifolium(L.) Sw. by various tissue culture techniques. Heliyon. 2016; 2(10): e00176. Published 2016 Oct 21. doi:10.1016/j.heliyon.2016.e00176
CrossRef - Huguenot C, Furneaux, M. T., Thottappilly, G., Rossel, H. W., Hamilton, R. I. Evidence that Cowpea aphid-borne mosaic and Blackeye cowpea mosaic viruses are two different potyviruses. Gen. Virol. 1993; 74: 335-340.
CrossRef - Green M R, Sambrook J. 2012. Molecular cloning – A laboratory manual I. Coldspring Harbor laboratory press, Coldspring Harbor, New York. 2021; 1881.
- Bag T K. Advances in the diagnosis and management of orchid viruses. In: Advances in plant Protection Sciences (Eds.) D. Prasad and Amerika Singh. Akansha Publishing House, New Delhi. 2004; pp. 343-361.
- Ajjikuttira P, Ho C L, Woon M H, Ryu K H, Chang C A, Loh C S, Wong S M. Genetic variability in the coat protein genes of two orchid viruses: Cymbidium mosaic virus andOdontoglossum ringspot virus. Virol. 2002; 147: 1943–1954.
CrossRef - Sudha D R, Usharani G. Incidence, and diagnosis of Cymbidium mosaic virus (CYMV) on orchids. J. Adv. Res. Biol. Sci. 2015; 2(3): 288–294.
- Bhat A I, Bhadramurthy V, Siju S, Hareesh P S. Detection and Identification of Cymbidium mosaic virus Infecting Vanilla (Vanilla planifolia Andrews) in India based on coat protein gene sequence relationships. Plant Biochemistry & Biotechnology. 2006; 15: 33-37.
CrossRef - Aiqing S, Lihua W, Yiping Z, Xiumei Y, Yi W, Dong Y, Wenhan L, Xuewei W. Establishment of a triplex TaqMan quantitative real-time PCR assay for simultaneous detection of Cymbidium mosaic virus, Odontoglossum ringspot virus and Cymbidium ringspot virus. Microbiol. 2023;14. DOI:10.3389/fmicb.2023.1129259
CrossRef - Jensen D D, Gold A H. A virus ringspot of Odontoglossum Orchid-Symptoms, transmission and electron microscopy. Phytopath. 1951; 41: 648-653.
- Sherpa A R, Hallan V, Zaidi A A. Cloning and sequencing of coat protein gene of an Indian Odontoglossum ringspot virus Acta. Virol. 2004; 48: 267–269.
- Hue J S, Ferreira S, Wang M, Xu M Q. Detection of Cymbidium mosaic virus, Odontoglossium ringspot virus, Tomato spotted wilt virus and Potyviruses infecting orchids in Hawaii. Plant Dis. 1993; 77: 464-468.
CrossRef - Kim S M, Ki E B, Sun H R, Choi Differentially expressed genes of Chenopodium amaranticolor in response to Cymbidium mosaic virus infection, Virus Res. 2016; 223: 43-51.
CrossRef - Lawson R H. Etiology of flower necrosis in cattleya orchids. 1970; 60: 36-40.
CrossRef - Wisler C G, Zettler F W, Purcifull D E. A serodiagnostic technique for detecting Cymbidium mosaic and Oontoglossum ringspot viruses. 1982; 72: 835-837.
CrossRef - Zheng, Y X, Chen C C, Chen Y K, Jan F J. Identification, and characterization of a potyvirus causing chlorotic spots on Phalaenopsis orchids. J. Plant Pathol. 2008; 121: 87–95.
CrossRef - Kim D H, Jeong R D, Choi S, Ju H J, Yoon J Y. Application of Rapid and Reliable Detection of Cymbidium Mosaic Virus by Reverse Transcription Recombinase Polymerase Amplification Combined with Lateral Flow Immunoassay. The plant pathology journal. 2022; 38(6): 665–672.
CrossRef - Rao X, Li Y, Sun J, Li X, Li M, Xiang M. Genetic Diversities of Cymbidium mosaic virus and Odontoglossum ringspot virus Isolates Based on the Coat Protein Genes from Orchids in Guangdong Province, China. Phytopathol. 2015;163. 324–329.
CrossRef - Yu H H, Wong S M. Synthesis of biologically active cDNA clones of Cymbidium mosaic potexvirus using a population cloning strategy. Arch Virol. 1998; 143: 1617–1620.
CrossRef - He Z, Jiang D, Liu A, Sang L, Li W, Li S. The complete sequence of Cymbidium mosaic virus from Vanilla fragrans in Hainan, China. Virus Genes. 2011; 42: 440 – 443.
CrossRef - Chen T, Pai H, Hou L, Lee S, Lin T, Chang C, Hsu F, Hsu Y, Lin N. Dual resistance of transgenic plants against Cymbidium mosaic virus and Odontoglossum ringspot virus. Scientific Reports, 2019; 9(1), 1-12. https://doi.org/10.1038/s41598-019-46695-7.
CrossRef - Petchthai U, Yee C S L, Wong, S M. Resistance to CymMV and ORSV in artificial microRNA transgenic Nicotiana benthamianaSci. Rep. 2018. 8, 9958. https://doi.org/10.1038/s41598-018-28388-9.
CrossRef - Safeer M M, Susha S Thara.Integrated management of Cymbidium mosaic disease in commercial Dendrobium orchids using root endophytic fungi Piriformospora indica, Cogent Food & Agriculture, 2022; 8:1, DOI: 10.1080/23311932.2022.2139848.
CrossRef

