Introduction
Electromagnetism and gravity are two fundamental forces that have played a pivotal role in the emergence of life on Earth, and they are believed to have ongoing effects on all living organisms. 1 Before the 1990s, sources of electromagnetic field radiations were limited, primarily consisting of a few radio and television transmitters. However, the past couple of decades have observed an extraordinary outburst of technological advancements, with global communication in particular experiencing rapid growth.2 Since the 1990s, the advent of wireless communication has led to a significant surge in the usage of cell phones, resulting in a substantial increase in the presence of electromagnetic pollution.3
The electromagnetic spectrum encompasses ionizing radiations, such as gamma and X‒rays, which have high frequency and short wavelengths, as well as non‒ionizing radiations, like radio and microwaves, which have low frequency and longer wavelengths. Over time, with technological advancements and increased communication sources, the radio frequencies used have also evolved. Initially, mobile phones were analog and operated in the 450‒900 MHz range. However, with progress, digital mobile phones with higher frequencies like 1800‒1900 MHz (2G), 2100 MHz (3G), 2300‒2600 MHz (4G), and more have been developed. The deployment of 5G networks, in particular, has attracted attention and debate. While 5G promises faster data transmission speeds and reduced latency, concerns have been raised regarding its potential health effects due to increased EMF exposure.4 The World Health Organization (WHO) has documented radio frequency (RF) electromagnetic radiations as possibly carcinogenic in nature.5
Electromagnetic field radiations (EMF‒r) have been extensively studied for their effects on various living organisms, including animals, humans, birds, micro‒organisms, and plants.6,7,8,9,10 While many scientists worldwide are researching the impact of EMF‒r on animals, there has been limited attention given to plants.11 Plants play a vital role in our environment as primary producers, converting inorganic nutrients into organic compounds and serving as a major source of oxygen. Consequently, it is of paramount importance to evaluate the effects of radio frequency (RF) EMF radiation on plants. Unlike mobile organisms, plants are immobile, which means they are continuously exposed to EMF‒r stress, making this research area crucial for understanding the potential impacts on the foundation of our ecosystems.
RF-EMF‒r has been reported to alter the growth, development and the activity of numerous enzymes, including those involved in reactive oxygen species (ROS) metabolism.12 Decline in germination percentage, seedling vigor index, and germination rate in Trigonella foenum‒graecum L. upon exposure of 900 MHz EMF‒r.13 Similarly, RF exposure also inhibits and delayed seed germination in Cicer arietinum L., and Phaseolus Aureus Roxb., respectively.14,15 In response to 900 MHz EMF-r exposure for 0.5, 1, 2 and h, rhizogenesis is severely impacted in Vigna radiata (L.) R. Wilczek along with elevation in the specific activities of stress related enzymes3. Further, it was reported that EMF-r exposure at 900 MHz on tomato resulted in transient decrease in the ATP content and adenylate energy charge (AEC) after 30 minutes of electromagnetic treatment.16 Given the widespread and continuous use of technology that emits EMF‒r, it is essential to understand the long‒term effects of EMF‒r exposure on human health and the environment.17 However, most of the studies conducted on exposure of EMF-r to plants are of short-term in nature. Moreover, study on EMF-r exposure to wheat crop growth, eco-physiology and carbohydrate metabolism is limited. Being a widely grown staple crop of the country, it is imperative to explore the impact of long-term exposure of EMF-r on the wheat crop growth and carbohydrate metabolism. In the current study, we observed the long-term exposure effects of EMF-r over a period of 30 and 60 days to investigate their impact on the growth, morphology, photosynthesis and carbohydrate metabolism of Triticum aestivum L. The hypothesis of the study was that the long-term exposure of EMF-r to wheat crop will reduce the growth by hampering the eco-physiological and metabolic activities. The objectives of the study were to (i) explore the response of crop growth and photosynthetic pigments, and (ii) carbohydrate metabolism of wheat crop exposed to 2850 MHz EMF‒r for 30 and 60 days.
Materials and methods
Plant material
Seeds of T. aestivum variety “SHARBATI‒MP” were surface sterilized with sodium hypochlorite solution (NaOCl, 0.1%, w/v) and then rinsed with distilled water. Three replications of each treatment were used, with one seed sown in each pot (⁓650 grams of sandy loam soil procured from a local nursery, Sector-26A, Chandigarh, India; soil pH= 6.5) in a completely randomized design (CRD). Plant samples from each treatment were stored at ‒20 ºC. All the chemicals and reagents were purchased from Sigma and Loba Chemie.
EMF‒r treatment
A vector signal generator (SMBV100A; R & S, Germany) within frequency range of 9 kHz─3.2 GHz was used to generate the radio frequency (RF) signals. An antenna and a radio frequency power amplifier (ZFL‒2500+; Minicircuits, USA) were equipped with the RF signal generator. Plant samples were subjected to 2850 MHz EMF‒r on every alternate day for 30 and 60 days (30 min per day) in an EMF‒shielded chamber. A radiation field meter (NBM 550) was used to measure the power flux density.
Plant morphology and biomass
After 30 and 60 days of exposure to 2850 MHz EMF‒r, plant samples were harvested. Using a measuring ruler, the root and shoot lengths were measured and recorded in centimeters (cm). The biomass of the plant samples was measured with the help of a weighing balance after oven‒drying the seedlings at 60 ºC for 72 h and the results were expressed in gram (g).
Total chlorophyll and carotenoid content
The photosynthetic pigment concentration was measured as per Hiscox and Israelstam.18 Briefly, 25 mg of leaf tissue was added to 4 ml of dimethyl sulfoxide (DMSO) and incubated at 60 ºC for 1 h. Absorbance was read at 470 nm, 645 nm, and 663 nm using a Shimadzu double beam spectrophotometer (UV‒1800, 240 V, Shimadzu Corporation, Kyoto, Japan). Following that, the chlorophyll and carotenoid content were determined and represented on a dry weight basis.19,20,21
Total water‒soluble carbohydrates and reducing sugars content
Extraction
Twenty mg of plant tissue was homogenized in 2 ml of distilled water and centrifuged at 15,000 g for 20 min at 4 ºC using a cold centrifuge (Sigma Inc., USA).
Procedure
Total carbohydrate content was assayed using the Loewus22 method. To 200 µl of anthrone reagent (0.2 g in 100 ml of H2SO4), 50 µl of plant extract was added. The intensity of the greenish‒blue color was recorded at 620 nm and represented as mg g‒1 f. wt. against the standard curve prepared with glucose. The methodology provided by Nelson23 was employed to determine the reducing sugars content. To 1 ml of reaction mixture (sodium carbonate, sodium sulphate, sodium potassium tartrate, and CuSO4.5H2O), 100 µl of plant extract, and 40 µl of distilled water were added. Samples were subjected to incubation for 20 min in hot water bath, after which 600 µl of ammonium molybdate and H2SO4 were added. Absorbance was measured at 520 nm against standard curve prepared with glucose, and presented as µg mg‒1 f. wt.
Acid and alkaline phosphatase activity
Twenty mg of plant tissue crushed in 2 ml of 50 mM tris‒maleate buffer and 13 mM mercaptoethanol (pH = 7.0), and centrifuged at 15,000 g (20 min, 4 ºC). The methodology described by Malik and Singh24 was used to assay acid and alkaline phosphatases activity using 0.3 ml of 0.1 M acetate buffer (pH = 5.0) and glycine sodium hydroxide buffer (pH = 10.5), respectively. To 50 µl of 0.2 M para‒nitrophenyl phosphate (p‒NPP) and 150 µl of enzyme extract were added. Samples were incubated at 37 ºC for 10 min, followed by addition of 2 ml of 0.3 M sodium carbonate solution. The absorbance was measured at 400 nm against the p‒nitrophenol standard curve, and represented as µg min‒1 mg‒1 protein.
Acid and alkaline invertase activity
Twenty mg of plant tissue was crushed in 2 ml of 50 mM tris‒maleate buffer containing 20 mM MgCl2 and 1 mM mercaptoethanol (pH = 7.5), and centrifuged at 15,000 g for 20 min at 4 ºC. Acid and alkaline invertase activities were evaluated as per the Nelson23 using 400 µl of 0.2 M sodium acetate buffer (pH = 4.8) and sodium phosphate buffer (pH = 8.0), respectively. After the addition of 100 µl of enzyme extract, samples were incubated at 45 ºC for 15 min. Absorbance was recorded at 620 nm after addition of 250 µl of Somogyi’s reagent and 250 µl of Nelson’s reagent against a calibration standard of glucose. The enzyme activities were represented as µg min‒1 mg‒1 protein.
α and β‒amylase activity
Twenty mg of plant tissue was crushed in 2 ml of 0.1 M sodium phosphate buffer (pH = 7.0) and centrifuged at 15,000 g (25 min, 4 ºC). α‒amylase activity was assayed as per the methodology given by Muentz.25 To 0.25 ml of enzyme extract, 0.5 ml of substrate solution (starch, potassium dihydrogen phosphate and calcium chloride), 50 µl EDTA and 1.5 ml of iodine solution were added. Absorbance was recorded at 630 nm using starch as standard, and represented as µg min‒1 mg‒1 protein. β‒amylase activity was examined as per the Bernfeld26 and later modified by Dure.27 To 0.25 ml of enzyme extract, 0.35 ml of substrate solution, and 50 µl of 0.1 M EDTA were added. Samples were incubated at 30 ºC for 30 min, followed by the addition of 0.5 ml of dinitrosalicylic acid (DNSA). Absorbance was recorded at 560 nm using maltose as standard and the results were represented as µg min‒1 mg‒1 protein.
Statistical analysis
The student’s t test was carried out using SPSS (version 16) to determine the significant differences between control and treatment groups at various levels of significance (P ≤ 0.05, P ≤ 0.01 and P ≤ 0.001). The data are presented as mean ± SE (standard error) of three replicates.
Results
EMF‒r impact on the growth and photosynthetic characteristics
The findings of the long-term exposure of EMF‒r on root and shoot length, biomass, and photosynthetic properties are summarized in Table 1. A significant (P ≤ 0.05) reduction by ⁓22 and ⁓26% in root length was observed in samples irradiated with EMF‒r for 30 and 60 days, respectively. Similarly, shoot length exhibited a reduction of ⁓24 and ⁓29%, respectively, compared to the control counterparts. The biomass of root and shoot showed a decline of ⁓33 and ⁓17% in 30‒days old plants and ⁓40 and ⁓20% in 60‒days old plants, respectively, when exposed to EMF‒r. Furthermore, the chlorophyll and carotenoid contents in treated samples exhibited a significant (P ≤ 0.05) reduction of ⁓24 and ⁓20% at 30 days, and ⁓31 and ⁓34% in 60‒days old plants, respectively, compared to their control counterparts.
Table 1: Effect of 2850 MHz EMF-r on the root and shoot length, biomass, and photosynthetic activity of T. aestivum plants harvested after 30 and 60 days.
|
Parameter studied |
Treatment group |
Triticum aestivum |
|
|
Root length (cm) |
30 days |
C |
46.43±2.34 |
|
T |
36.30±0.78* |
||
|
60 days |
C |
70.57±1.98 |
|
|
T |
52.47±1.52** |
||
|
Shoot length (cm) |
30 days |
C |
33.50±1.50 |
|
T |
25.43±0.75** |
||
|
60 days |
C |
60.43±2.54 |
|
|
T |
42.90±2.07** |
||
|
Root biomass (g) |
30 days |
C |
0.16±0.00 |
|
T |
0.11±0.01** |
||
|
60 days |
C |
0.22±0.02 |
|
|
T |
0.14±0.01* |
||
|
Shoot biomass (g) |
30 days |
C |
0.45±0.02 |
|
T |
0.34±0.02* |
||
|
60 days |
C |
0.78±0.03 |
|
|
T |
0.62±0.02** |
||
|
Total chlorophyll content (mg g-1 d.wt.) |
30 days |
C |
12.04±0.22 |
|
T |
9.13±0.60* |
||
|
60 days |
C |
11.96±0.70 |
|
|
T |
8.51±0.91* |
||
|
Total carotenoid content (mg g-1 d.wt.) |
30 days |
C |
3.84±0.10 |
|
T |
3.09±0.03** |
||
|
60 days |
C |
3.90±0.20 |
|
|
T |
2.65±0.24* |
||
The data are presented as mean ± SE (standard error) of three replicates. Asterisks indicate difference among treatment groups based on student’s t test at different levels of significance (P ≤ 0.05*, P ≤ 0.01** and P ≤ 0.001***). C and T represent control and treatment groups, respectively.
EMF‒r Impact on carbohydrate metabolism
Upon exposure to EMF-r, T. aestivum plant samples exhibited a significant decrease (P ≤ 0.05) in the total carbohydrate and reducing sugars content (Fig. 1). Compared to control, the carbohydrate content of EMF‒r exposed shoots exhibited a considerable reduction of ⁓24% and ⁓30% in shoots and ⁓21% and ⁓44% in the roots of the 30 and 60‒days old plants, respectively. Similarly, reducing sugar content significantly declined by ⁓35 and ⁓33% in shoots, and ⁓28% and ⁓42% in roots, respectively. The acid phosphatase activity exhibited a decline of ⁓28 and ⁓42%, and ⁓38% and ⁓45% in shoots and roots, respectively. However, alkaline phosphatase activity was found to be reduced by ⁓38 and ⁓40%, and ⁓56% and ⁓65% after 30- and 60-days exposure to EMF-r in shoots and roots, respectively, compared to their control counterparts (Fig. 2).
The α‒amylase activity decreased significantly by ⁓30 and ⁓32% in shoots, ⁓58 and ⁓53% in roots harvested after 30 and 60 days, respectively. However, β‒amylase activity declined by ⁓34 and ⁓49% in shoots, and ⁓57 and ⁓50% in roots compared to the control (Fig. 3). Furthermore, the activity of acid invertases significantly decreased by ⁓33 and ⁓35% in shoots, and ⁓32 and ⁓40% in roots, while alkaline invertases activity declined by ⁓28 and ⁓25% in shoots, and ⁓38 and ⁓42% in roots after 30 and 60 days of irradiation, respectively (Fig. 4).
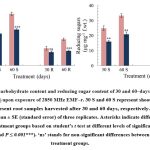 |
Figure 1: Total carbohydrate content and reducing sugar content of 30 and 60‒days old T. aestivum shoots (S) and roots (R) upon exposure of 2850 MHz EMF‒r. |
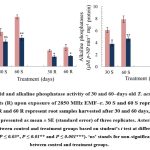 |
Figure 2: Acid and alkaline phosphatase activity of 30 and 60‒days old T. aestivum shoots (S) and roots (R) upon exposure of 2850 MHz EMF‒r. |
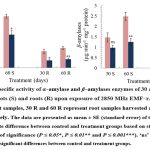 |
Figure 3: The specific activity of α‒amylase and β‒amylases enzymes of 30 and 60‒days old T. aestivum shoots (S) and roots (R) upon exposure of 2850 MHz EMF‒r. |
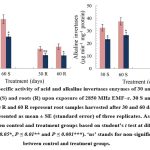 |
Figure 4: The specific activity of acid and alkaline invertases enzymes of 30 and 60‒days old T. aestivum shoots (S) and roots (R) upon exposure of 2850 MHz EMF‒r. |
Discussion
The findings of the current study revealed that exposure to EMF‒r adversely affected both the root and shoot lengths of T. aestivum, especially when subjected to longer duration of 60 days, resulting in a hindrance to plant growth. This decline in root and shoot length of EMF‒r irradiated samples aligns with previous research findings.3,14,28 In general, the plant’s response to EMF‒r varies considerably and lacks consistency. It depends on factors such as exposure type, its intensity, duration, plant’s genotype and many more.29 The decrease in photosynthetic pigments observed following EMF‒r exposure indicates that EMF‒r may disrupt the photosynthetic machinery.30,31
In the current investigation, we observed a significant decrease in the total carbohydrate levels during the 30 and 60 days of exposure duration. It is worth noting that no prior reports are available that have documented the long-term exposure effects of EMF‒r on carbohydrate metabolism. Nevertheless, in a similar study, an initial increase followed by decrease in carbohydrate content at 2 and 4 h of EMF‒r exposure duration was observed.32 A reduction in the carbohydrate content in V. radiata after one week of EMF‒r exposure was observed.11 On the contrary, elevation in total soluble sugar and carbohydrate levels of Zea mays L. and T. aestivum was reported upon exposure of 945 MHz and 50 Hz EMF‒r, respectively.33,34 The carbohydrate content in plants can vary significantly due to the intricate interplay between the plant and its surrounding environment. To adapt to adverse abiotic conditions, plants store various metabolites, and the specific carbohydrates that can vary depending on the stress conditions.35 However, when stress levels become more intense, as observed in the current study, there tends to be a decline in carbohydrate content.36
The reduction in carbohydrate content could potentially be attributed to increased activity levels of enzymes responsible for breaking down starch i.e., α‒amylases and β‒amylases, or a decline in photosynthetic pigments, leading to the absence of newly synthesized carbohydrates due to damage in photosynthetic machinery, as observed in the current investigation. Starch, produced during the photosynthesis process, serves as a temporary reserve of fixed carbon, while sucrose is the primary source of energy. Within plant cells, starch is enzymatically broken down by α‒ and β‒amylases, resulting in the formation of glucose and maltose, either individually or in combination, which are subsequently utilized in the glycolytic pathway or for sucrose synthesis.37,38
A significant decline in the α‒ and β‒amylases activities was observed upon exposure of 2850 MHz EMF‒r in both 30 and 60‒days old plant samples. Our findings corroborate with Mahajan et al.36 where the authors observed reduction in the activity of amylases in Z. mays roots. The possible reason for decline in amylase activity could be due to cellular damage and oxidative stress in exposed samples in response to EMF‒r. Enzymes, including amylases, may become damaged or inactivated by reactive oxygen species generated during stress responses.39 Invertases play a key role in regulating the distribution of sucrose to various cellular compartments, by breaking down sucrose into glucose and fructose. They also play a significant role in adapting to environmental stress conditions, such as maintaining osmotic pressure, contributing to defense responses, and participating in cell and development.40 The reduced activity of acid and alkaline invertases implies interference with the allocation of carbon resources, as well as the regulation of growth and development.41 In the present study, a reduction in invertases activity was observed in plants exposed to long-term EMF‒r exposure. This subsequently restricts the availability of sucrose within the cells. These findings corroborate with the results obtained by Sidhu et al.42 who demonstrated a decline in the production of invertases in Coronopus didymus L. when exposed to different lead (Pb) concentrations.
Acid and alkaline phosphatases, play a key role in managing the distribution of inorganic phosphorus (Pi) to various parts of developing plants, to maintain the pace of growth and the efficiency of physiological processes.43 In our current investigation, a significant decline in the activity of both acid and alkaline phosphatases with longer exposure periods was observed. The reduction in phosphatase activity during prolonged exposure results in reduced Pi availability, thereby leading to diminished growth.44
Conclusions
The present study offers concrete evidence on the impact of prolonged exposure to 2850 MHz EMF‒r on T. aestivum at varying durations. The findings suggest that EMF‒r negatively affects plant physiology, leading to disruptions in normal processes during different growth stages. Notably, EMF‒r exposure impedes growth by disrupting photosynthetic pigments and carbohydrate metabolism. These effects are directly linked to the duration of exposure, with more significant damage occurring during longer exposure periods, emphasizing the influence of EMF intensity on biological outcomes. The increasing frequency and exposure duration may hamper the crop metabolism and nutritional value in the coming time which needs attention.
Acknowledgments
The authors are grateful to the Council of Scientific and Industrial Research (CSIR), New Delhi (India) for providing the research grant that supported this study.
Funding Source
AP is thankful to the Council for Scientific and Industrial Research (CSIR) for providing Research Fellowship as Senior Research Fellow.
Conflict of Interest
Authors declare that they do not have any conflict of interest.
Authors’ Contribution
AP: Experimentation, Data collection, Calculation, Analysis, Graph Preparation, Initial Drafting of manuscript; DRB: Conception, Research Design, Review and finalization, Supervision; SK: Research Design, Data curation, Statistical analysis, Review and finalization; RS: Statistical analysis, Review and finalization.
Data Availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Informed consent statement
All the authors have gone through the final version of the manuscript and agreed with the submission of manuscript to the journal. The manuscript has been given full consent for further processing by the journal.
References
- Delgado, J. M. R. Biological effects of extremely low frequency electromagnetic fields. J Bioelec. 1985;4(1):75‒92.
- Kaur, S., Vian, A., Chandel, S., Singh, H. P., Batish, D. R., Kohli, R. K. Sensitivity of plants to high frequency electromagnetic radiation: cellular mechanisms and morphological changes. Rev Environ Sci Biotechnol. 2021;20(1):55–74.
- Singh, H. P., Sharma, V. P., Batish, D. R., Kohli, R. K. Cell phone electromagnetic field radiations affect rhizogenesis through impairment of biochemical processes. Environ Monit Assess. 2012;184:1813‒1821.
- Elbasheir, M. S., Saeed, R. A., Ibrahim, A. A., Edam, S., Hashim, F., Fadul, S. M. A review of EMF radiation for 5G mobile communication systems. IEEE Asia‒Pacific Conference on Applied Electromagnetics 2021, December pp 1‒6.
- IARC classifies radiofrequency electromagnetic fields as possibly carcinogenic to humans (31 May, 2011). \ Accessed 25 December 2023
- Tran, N. T., Jokic, L., Keller, J., Geier, J.U., Kaldenhoff, R. Impacts of radio‒frequency electromagnetic field (RF‒EMF) on lettuce (Lactuca sativa)—evidence for RF‒EMF interference with plant stress responses. Plants. 2023;12(5):1082.
- Treder, M., Müller, M., Fellner, L., Traynor, K., Rosenkranz, P. Defined exposure of honey bee colonies to simulated radiofrequency electromagnetic fields (RF‒EMF): Negative effects on the homing ability, but not on brood development or longevity. Sci. Total Environ. 2023;896:165211.
- Levitt, B. B., Lai, H. C., Manville, A. M. Effects of non‒ionizing electromagnetic fields on flora and fauna, part 2 impacts: how species interact with natural and man‒made EMF. Rev Environ Health. 2022;37(3):327‒406.
- Kundu, A., Vangaru, S., Bhattacharyya, S., Mallick, A. I., Gupta, B. Electromagnetic irradiation evokes physiological and molecular alterations in rice. 2021;42(2):173‒185.
- Vienne‒Jumeau, A., Tafani, C., Ricard, D. Environmental risk factors of primary brain tumors: A review. Rev Neurol. 2019;175(10):664‒678.
- Sharma, V. P., Singh, H. P., Batish, D. R., Kohli, R. K. Cell phone radiations affect early growth of Vigna radiata (mung bean) through biochemical alterations. Z Naturforsch C. 2010;65(1-2):66‒72.
- Chandel, S., Kaur, S., Singh, H. P., Batish, D. R., Kohli, R. K. Exposure to 2100 MHz electromagnetic field radiations induces reactive oxygen species generation in Allium cepaJ Microsc Ultrastruct. 2017;5(4):225‒229.
- Sharma, S., Bahel, S., Kaur, J. K. Evaluation of oxidative stress and genotoxicity of 900 MHz electromagnetic radiations using Trigonella foenum‒graecum test system. Protoplasma. 2023;260(1):209‒224.
- Qureshi, S. T., Memon, S. A., Abassi, A. R., Sial, M. A., Bughio, F. A. Radiofrequency radiations induced genotoxic and carcinogenic effects on chickpea (Cicer arietinum) root tip cells. Saudi J. Biol. Sci. 2017;24(4):883‒891.
- Sharma, V. P., Singh, H. P., Kohli, R. K. Effect of mobile phone EMF on biochemical changes in emerging seedlings of Phaseolus aureusEcoscan. 2009;3(3-4):211‒214.
- Roux, D., Vian, A., Girard, S., Bonnet, P., Paladian, F., Davies, E., Ledoigt, G. High frequency (900 MHz) low amplitude (5 V m− 1) electromagnetic field: a genuine environmental stimulus that affects transcription, translation, calcium and energy charge in tomato. Planta. 2008;227:883‒
- Franczak, A., Waszkiewicz, E. M., Kozlowska, W., Zmijewska, A., Koziorowska, A. Consequences of electromagnetic field (EMF) radiation during early pregnancy‒androgen synthesis and release from the myometrium of pigs in vitro. Anim Reprod Sci. 2020;218:106465.
- Hiscox, J. D., Israelstam, G. F. A method for the extraction of chlorophyll from leaf tissue without maceration. Canad J Bot. 1979;57(12):1332‒1334.
- Arnon, D. I. Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris. Plant Physiol. 1949;24:(1)1‒15.
- Lichtenthaler, H. K., Wellburn, A. R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc Trans. 1983;11(5):591-592.
- Batish, D. R., Singh, H. P., Setia, N., Kaur, S., Kohli, R. K. Chemical composition and phytotoxicity of volatile essential oil from intact and fallen leaves of Eucalyptus citriodora. Z. fur Naturforsch. 2006;61(7-8):465-471.
- Loewus, F. A. Improvement in anthrone method for determination of carbohydrates. Anal Chem. 1952;24(1):219‒219.
- Nelson, N. A photometric adaptation of the Somogyis method for the determination of reducing sugar. Anal Chem. 1944;31:426‒428.
- Malik, C. P, Singh, M. B. Estimation of polyphenols. In: Plant enzymology and histo‒enzymology. New Delhi, India: Kalyani Publishers; 1980:286‒288.
- Muentz, K. Isoenzymes of α‒amylase during pod development of field beans. Phytochemistry.1977;16(10):1491‒1494.
- Bernfeld, P. Amylase, α and β. Meth. Enzymol.1955;1:149‒158.
- Dure, L.S. Site of origin and extent of activity of amylases in maize germination. Plant Physiol. 1960; 35(6):925-935.
- Johal, N., Batish, D., Pal, A., Chandel, S., Pal, M. Investigating the effects of 2850 MHz electromagnetic field radiations on the growth, germination and antioxidative defense system of chickpea (Cicer arietinum) seedlings. Russ J Plant Physiol. 2022;69(6):1‒8.
- Halgamuge, M. N., Yak, S. K., Eberhardt, J. L. Reduced growth of soybean seedlings after exposure to weak microwave radiation from GSM 900 mobile phone and base station. Bioelectromagnetics. 2015;36(2):87‒95.
- Tang, C., Yang, C., Yu, H., Tian, S., Huang, X., Wang, W., Cai, P. Electromagnetic radiation disturbed the photosynthesis of Microcystis aeruginosa at the proteomics level. Sci Rep. 2018;8(1):479.
- Senavirathna, M. D. H. J., Takashi, A., Kimura, Y. Short‒duration exposure to radiofrequency electromagnetic radiation alters the chlorophyll fluorescence of duckweeds (Lemna minor). Electromagn. Biol. 2014;33(4):327‒334.
- Kumar, A., Singh, H. P., Batish, D. R., Kaur, S., Kohli, R. K. EMF radiations (1800 MHz)‒inhibited early seedling growth of maize (Zea mays) involves alterations in starch and sucrose metabolism. Protoplasma. 2016;253:1043‒1049.
- Khalafallah, A. A., Sallam, S. M. Response of maize seedlings to microwaves at 945 MHz. Rom J Biophys. 2009;19(1): 49‒62.
- Hanafy, M. S., Mohamed, H. A., Abd El‒Hady, E. A. Effect of low frequency electric field on growth characteristics and protein molecular structure of wheat plant. Rom J Biophys 2006;16(4):253‒271.
- Trouvelot, S., Héloir, M. C., Poinssot, B., Gauthier, A., Paris, F., Guillier, C., Combier, M,, Trdá, L., Daire, X., Adrian, M. Carbohydrates in plant immunity and plant protection: roles and potential application as foliar sprays. Front. Plant Sci. 2014;5:592.
- Mahajan, P., Singh, H. P, Batish, D. R., Kohli, R. K. Cr (VI) imposed toxicity in maize seedlings assessed in terms of disruption in carbohydrate metabolism. Biol Trace Elem Res. 2013;156:316‒322.
- Das, R., Kayastha, A. M. An Overview on Starch Processing and Key Enzymes. In: Wu J, Xia W (eds) Industrial Starch Debranching Enzymes. Singapore: Springer; 2023:1‒20.
- Stanley, D., Farnden, K. J., MacRae, E. A. Plant α‒amylases: functions and roles in carbohydrate metabolism. Biologia. 2005;60:65‒71.
- Rochalska, M., Grabowska, K. Influence of magnetic fields on the activity of enzymes: alpha‒and beta‒amylase and glutathione S‒transferase [GST] in wheat plants. Int. Agrophysics. 2007;21(2):185‒188.
- Wang, L., Zheng, Y., Ding, S., Zhang, Q., Chen, Y., Zhang, J. Molecular cloning, structure, phylogeny and expression analysis of the invertase gene family in sugarcane. BMC Plant Biol. 2017;17:1‒20.
- Hütsch, B. W., Jung, S., Schubert, S. Comparison of salt and drought‐stress effects on maize growth and yield formation with regard to acid invertase activity in the kernels. J Agron Crop Sci. 2015;201(5):353‒367.
- Sidhu, G. P. S., Singh, H. P., Batish, D. R., Kohli, R. K. Alterations in photosynthetic pigments, protein, and carbohydrate metabolism in a wild plant Coronopus didymus (Brassicaceae) under lead stress. Acta Physiol. Plant. 2017;39:1‒9.
- Duff, S. M., Sarath, G., Plaxton, W. C. The role of acid phosphatases in plant phosphorus metabolism. Physiol Plant. 1994;90(4):791‒800.
- Mishra, S., Dubey, R. S. Changes in phosphate content and phosphatase activities in rice seedlings exposed to arsenite. Braz. J. Plant Physiol. 2008;20(1): 19‒28.

