Introduction
Industrialization, urbanization, economic growth and associated increase in energy demands have resulted in a profound deterioration of air quality in developing countries like India. Oxides of nitrogen and sulphur and fly-ash constitute as the major proportions for the gaseous and particulate emissions from industries and automobile. The exposure of these pollutants to the leaves cause a reduction in the concentration of their photosynthetic pigments viz., chlorophyll and carotenoids, which affects the plant productivity, germination of seeds, length of pedicles, and number of flowers inflorescence.1 Chlorophyll is the principal photoreceptor in photosynthesis, the light-driven process by which carbon dioxide is “fixed” to yield carbohydrates and oxygen. While carotenoid is a class of natural fat-soluble pigment found principally in plants, algae and photosynthetic bacteria, where they play a critical role in the photosynthetic process2 and also protect chlorophyll from photoxidative destruction.3 When plants are exposed to the environmental pollution above the normal physiologically acceptable range, photosynthesis gets inactivated.4 Since, the plants leaf samples used for this experiment were constantly exposed to air pollutants (polluted area-industrial area, automobile area & less polluted area–jungle, they had absorbed,5 accumulated and integrated pollutants on their surface and showed specific response. Hence, plants can be used as bioindicators in various field of research.6
Bhopal City of Lakes is an important tourist centre and industrially developing city. It is densely populated and has heavy vehicular traffic. Air pollution has increased tremendously that is affecting the proper growth of plants in its vicinity. The rapid addition of toxic substances to environment is responsible for altering the ecosystem.7 Plants growing in heavy trafficular area are thus exposed to variety of pollutants such as SMP, RSMP, NoX , & So2 etc.
Materials and Methods
Present investigation deals with comparative study of under heavy trafficular pollution with those growing in less or unpolluted areas. For this purpose leaf samples of Azadirachta indica (Neem), Nerium oleander(Kaner), Mangifera indica(Mango) and Dalbergia sissoo (Sheeshame) were collected from highly polluted and less or unpolluted area.
Study Area and Sample Collection
Four sites were selected for polluted area as well as for less or non polluted area from Bhopal City district and capital of Madhya Pradesh, situated at 23° 15′ 0″ N, 77° 25′ 0″ E in India. For polluted area samples were collected from Mandideep and Govindpura as industrial area and DIG Banglow (situated near Union Carbide India limited pesticide plant responsible for Bhopal Gas Tragedy), Chetak Bridge as highly traffic area. For non polluted or less polluted area we selected Kaliasot Jungle, VIP Road, Indian Institute of Soil Science & Barkatullah University.
Determination of Chlorophyll Content
50 mg of fresh leaf tissue was weighed accurately; Chlorophyll was extracted by crushing leaf and suspended in test tubes containing 10 ml of dimethyl sulphoxide (DMSO). Test tubes were incubated at 60° C – 65° C for 4 hour in a hot air oven. The supernatant was decanted and the chlorophyll extract was transferred to a cuvette and the absorbance was read in a spectrophotometer at 645 and 663 nm against DMSO blank8 Chlorophyll a, b, total chlorophyll and chlorophyll a/b ratio were calculated by using formulae given by.9
Results And Discussion
Photosynthetic Pigment Changes
Variations in physiological characteristics of selected plant species exposed to cement dust pollutants are given in Table 1, 2,3 and 4 (Fig. 1). The results obtained with polluted and non polluted Azadirachta indica, Nerium oleander, Mangifera indica and Dalbergia sissoo were compared. In general, plants showed a decrease in photosynthetic pigments due to air pollution. Azadirachta indica, Nerium oleander, Mangifera indica and Dalbergia sissoo showed a significant reduction in total chlorophyll content, chlorophyll ‘a’ and chlorophyll ‘b’ in the study period. But there is no significant change in total carotenoids of the selected plant species.
Azadirachta Indica
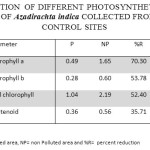 |
Table 1: Concentration of Different Photosynthetic Pigments (mg G-1) in the Leaves of Azadirachta Indica Collected from Polluted and Control Sites. Click here to View table |
Mangifera Indica
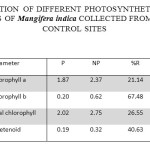 |
Table 2: Concentration of Different Photosynthetic Pigments (mg G-1) in the Leaves of Mangifera Indica Collected from Polluted and Control Sites. Click here to View table |
Nerium Oleander
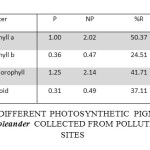 |
Table 3: Concentration of Different Photosynthetic Pigments (mg G-1) in the Leaves of Nerium Oleander Collected from Polluted and Control Sites. Click here to View table |
Dalbergia Sissoo
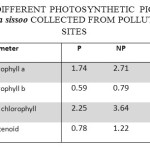 |
Table 4: Concentration of Different Photosynthetic Pigments (mg G-1) in the Leaves of Dalbergia Sissoo Collected from Polluted and Control Sites. Click here to View table |
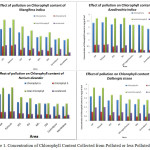 |
Figure 1: Concentration of Chlorophyll Content Collected from Polluted or less Polluted area. Click here to View figure |
Azadirachta Indica
The concentration of Chl ‘a’ in the leaves of Azadirachta indica at polluted sites was recorded as 0.49±0.09 mg/g which was 1.65±0.27 mg/g at the control site. Thus a reduction of 70.3% in Chlorophyll ‘a’ was recorded in the samples from the polluted sites in comparison to control. The concentration of Chl ‘b’ was 0.28±0.10 mg/g in the leaf samples collected from polluted sites while it was 0.69±0.09 mg/g in the samples from control site. The polluted sites sample thus had 53.78% less Chl ’b’ content. Total chlorophyll content was 1.04±0.05 mg/g and 2.19±0.05 in the leaf samples collected from polluted and control site respectively. Thus, there was a reduction of 52.4% in the total chlorophyll content in the samples from polluted site. The concentration of total carotenoids in the leaf samples from polluted and control site was recorded as 0.36±0.06 mg/g and 0.56±0.16 mg/g respectively with a reduction of 35.71% in leaf samples from polluted sites.
Mangifera Indica
The concentration of Chl ‘a’ in the leaves of Mangifera indica at polluted sites was recorded as 1.87±0.35 mg/g which was 2.37±0.42 mg/g at the control site. Thus a reduction of 21.14% in Chlorophyll ‘a’ was recorded in the samples from the polluted sites in comparison to control. The concentration of Chl ‘b’ was 0.20±0.04 mg/g in the leaf samples collected from polluted sites while it was 0.62±0.14 mg/g in the samples from control site. The polluted sites sample thus had 67.48% less Chl ‘b’ content. Total chlorophyll content was 2.02±0.46 mg/g and 2.75±0.65 in the leaf samples collected from polluted and control site respectively. Thus, there was a reduction of 26.55% in the total chlorophyll content in the samples from polluted site. The concentration of total carotenoids in the leaf samples from polluted and control site was recorded as 0.19±0.09 mg/g and 0.32±0.12 mg/g respectively with a reduction of 40.63% in leaf samples from polluted sites.
Nerium Oleander
The concentration of Chl ‘a’ in the leaves of Nerium oleander at polluted sites was recorded as 1.00±0.35 mg/g which was 2.02±0.32 mg/g at the control site. Thus a reduction of 50.37% in Chlorophyll ‘a’ contentwas recorded in the samples from the polluted sites in comparison to control. The concentration of Chl ‘was 0.36±0.14 mg/g in the leaf samples collected from polluted sites while it was 0.47±0.24 mg/g in the samples from control site. The polluted sites sample thus had 24.51% less Chl ‘b’ content. Total chlorophyll content was 1.25±0.46 mg/g and 2.14±0.35 in the leaf samples collected from polluted and control site respectively. Thus, there was a reduction of 41.71% in the total chlorophyll content in the samples from polluted site. The concentration of total carotenoids in the leaf samples from polluted and control site was recorded as 0.31±0.08 mg/g and 0.49±0.11 mg/g respectively with a reduction of 37.11% in leaf samples from polluted sites.
Dalbergia Sissoo
The concentration of Chl ‘a’ in the leaves of Dalbergia sissoo at polluted sites was recorded as 1.74±0.35 mg/g which was 2.71±0.42 mg/g at the control site. Thus a reduction of 35.98% in chl ‘a’ content was recorded in the samples from the polluted sites in comparison to control. The concentration of Chl ‘b’ was 0.59±0.14 mg/g in the leaf samples collected from polluted sites while it was 0.79±0.24 mg/g in the samples from control site. The polluted sites sample thus had 24.66% less Chl ‘b’ content. Total chlorophyll content was 2.25±0.46 mg/g and 3.64±0.65 in the leaf samples collected from polluted and control site respectively. Thus, there was a reduction of 38.32% in the total chlorophyll content in the samples from polluted site. The concentration of total carotenoids in the leaf samples from polluted and control site was recorded as 0.78±0.08 mg/g and 1.22±0.11 mg/g respectively with a reduction of 36.48% in leaf samples from polluted sites.
Air pollutants, fly ash and dust emissions have a profound impact on the concentration of different photosynthetic pigments. Polluted and dusted leaf surface is responsible for reduced photosynthetic and thereby causing reduction in chlorophyll content.10 The similar impact of air pollutants in the concentration of chlorophyll contents have been reported by a number of other works.11,12,13,14,15,16,17 In the present study the highest decrease in total chlorophyllwas in Azadirachta indica (52.40%) followed by Nerium oleander (41.71%) Delbergia sissoo (38.32%) and Mangifera indica (26.55%). The two way ANOVA showed that the reduction in chlorophyll content of Azadirachta indica Nerium oleander Delbergia sissoo and Mangifera indica were significant at 0.05% level.
Total Average Amount of Assimilating Pigments
Total average amount of assimilating pigments (a+b+c) in the control plant leaves of Azadirachta indica was maximum reduction (53.27%) compared with Mangifera indica (43.08%) Nerium oleander (37.33%) and Delbergia sissoo (31.48%). The chlorophyll a + b, carotenodic pigments, (a +b/c) ratio had extremely low values compared to the control because of significant drop of both types of chlorophyll as well as the increase of carotenoidic pigments. It indicates the plant species are under stress and also had damage due to pollution.
The photosynthetic pigments are the most likely to be damaged by air pollution. Chlorophyll pigments exist in highly organized state, and under stress they may undergo several photochemical reactions such as oxidation, reduction, pheophytinisation and reversible bleaching.18 Hence any alteration in chlorophyll concentration may change the morphological, physiological and biochemical behaviour of the plant. Air pollution-induced degradation in photosynthetic pigments was also observed by a number of workers.19,20,21 In both the plants chlorophyll a and chlorophyll b content were reduced significantly at polluted site.
The results of this study indicated a decline in chlorophyll content in trees growing in industrial area. The reduction in chlorophyll content is due to degradation of chlorophyll into phaeophytin by the loss of magnesium ions. Chlorophyll content may differ in different period of time under different conditions of pollution stress and different meteorological conditions. Thus it is concluded that in the study area there is need to develop green belt for the betterment of environment and human being. On the basis of this study, it could be concluded that growth of plants was found to be affected by cement dust, which might be due to the presence of different toxic pollutants in cement dust. The phonological behavior of Azadirachta indica was found to be highly affected than Mangifera indica, Nerium oleander and Delbergia sissoo. It is clear that the air pollution caused by industries and automobile smoke are operative ecological factor causing deterioration in the quality of our environment.22 From the correlated interpretation of the obtained data, one can conclude that, beside particular manifestations of interactions between atmospheric pollutants (gas and solid) and vegetations, there is a common series of manifestations, as a general response to the stress caused by pollutants aggressions, regardless of their chemical nature. Under the influence of solid and gas polluting agents the average photosynthetic pigment amount drops, the values being most often correlated to the pollutants. Further investigations are necessary, in order to study the variation of these pigments in the control leaves along all vegetative season and to anticipate more accurately the possible ‘answers’ that the vegetation might have when subjected to a chronic aggression from atmospheric polluting agents.
Acknowledgements
The authors are thankful to Institute for Excellence in Higher Education, Bhopal for their financial assistance to carry out a research work on Chlorophyll Content.
References
- Nithamathi, C. P., and Indir a, V., “Imp act of air p ollution On Ceasalpinia sepiaria Linn. in Tuticorin City” ., Indian Journal of Environment and Ecop lanning, 10 (1),449–452. (2005).
- Ong, A.S.H., and Tee, E.S.,“Natural sources of Carotenoids from plants and oils”, Meth. Enzymol, vol. 213, pp. 142-167, (1992).
CrossRef - Siefermann-Harms, D., “The light harvesting and protective function of carotenoids in photosynthetic membranes”, Physiologia Plantarum, vol. 69, pp. 561-568, (1987).
CrossRef - Miszalski, Z., and Mydlarz, J., “SO2 influence on photosynthesis of tomato plants at different CO2 concentrations”, Photosynthetica, vol.24, pp. 2-8, (1990).
- Govindaraju., Ganeshkumar.R.S Suganthi.P Muthukumaran.V.R, Visvanathan.P” Impact Assessment of Air Pollution Stress on Plant Species through Biochemical Estimations” ,(2010).
- Joshi, O.P., Wagela, D.K., and Pawar., K., “Urban air pollution effect on two species of cassia.” poll res. 16(1):1-3, (1997).
- Niragau, J. D., and Davidson, C. l., Toxic metals in the atmosphere, Jon Wiley and Sons, Newyork, (1986).
- Hiscox, J.D., Israelstam, G.F., a method for the extraction of chlorophyll from leaf tissue without maceration. canadian journal of botany 57: 1332-1334, (1979).
- Arnon, D.I., copper enzymes in isolated chloroplasts, polyphenoxidase in beta vulgaris. plant physiology 24: 1-15, (1949).
- Kalyani, V., and Singaracharya M.A., “Biomonitoring of air pollution in Warangal city (A.P.)”. Act Botanica indica 23:21-23, (1995).
- Nikova, D. and Dushkova, P. 919780. Gorshostoparnska Nauka. 3: 3-8.
- Rabe, R. and Kareeb, K. H., Enzyme activites and chlorophyll and protein content in plants as indicator of air pollution. Environ. Pollut. 16:119-137, (1979).
CrossRef - Dubey, P. S. and Pawar, K., Air pollution and plant response- Review. In:Perspective in environment botany (Eds.:D. N. Rao,K. J. Ahamed. M. Yunus and S. N. Singh). Print House,Lucknow. 1: 101-118, (1985).
- Shah, F. H., Ilahi, I. and Rashid, A., Effect of cement dust on the chlorophyll contents, stomatal clogging and biomass of some selected plants. Pakistan J. Sci. Indust. Research. 32(8): 542-545, (1989).
- Saxena, R. M., Effect of motorway pollution on seed health of some vegetable crops. Ind. J. Environ. Hlth. 33: 385-387, (1991).
- Swami, A. D., Bhatt and Joshi, P. C., Effect of automobile pollution on Sal(Shorea robusta)and Rohini (Mallotus phillipinesis) at Asarori, Dehradun. Him. J. Environ. Zool. 18: 57-61, (2004).
- Tripathi, A. K. and Gautam, M. Biochemical parameters of plants as indicators of air pollution. J. Environ. Biol. 28: 127-132, (2007).
- Puckett, K.J., Nieboer, E., Flora W.P., and Richardson, D.H.S. “Sulphur dioxide: Its effect on photosynthetic 14C fixation in lichens and suggested mechanism of phytotoxicity” The New Phytologist, vol. 72, pp. 141–154.
CrossRef - Bansal, S., “Studies on the effect of certain atmospheric pollutants on fruit diseases of Lycopersicon esculentum Mill. Caused by Alternaria alternate”, PhD thesis, Bhopal, Bhopal University, (1988).
- Singh, N., Singh, S.N., Srivastava., K., Yunus, M., Ahmad, K.J., and Sharma S. C., et al., “Relative sensitivity and tolerance of some Gladiolus cultivars to sulphur dioxide”, Annals of Botany, vol. 65, pp. 41–44, (1990).
CrossRef - Sandelius, A.S., Naslund., K., Carlson, A.S., Pleijel, H., and Sellden, G., “Exposure of spring wheat (Triticum aestivum) to ozone in open top chambers. Effects on acyl lipid composition and chlorophyll content of flag leaves”, The New Phytologist, vol. 131, pp. 231–239, ( 1995).
CrossRef - Shah, F. H., Ilahi, I. and Rashid, A., Effect of cement dust on the chlorophyll contents, stomatal clogging and biomass of some selected plants. Pakistan J. Sci. Indust. Research. 32(8): 542-545, (1989).
- Britton, G., “Structure and properties of carotenoids in relation to function”, FASEB J., vol. 9, pp. 1551-1558, (1995).
