Introduction
Seed germination is usually the most critical stage in seedling establishment determining successful crop production.1, 2 The germination of seed is a complex process depending on the genetic and environmental factors; such as temperature, light and salinity.3 Salinity adversely affects the plant growth and development, hindering seed germination.4 There are two basic ways in which salinity affects the plants. First high salt concentration in soil made it harder for plant roots to extract water from the soil. This is purely the result of osmosis, the movement of water across a semi-permeable membrane as in a plant cell, from an area of high water potential (low salt concentration) to an area of low water potential (high salt concentration). When the concentration of soil-water salt stress above threshold water will tends to flow out of the plant. If plants had no way of regulating this process they would quickly dehydrate and die. Secondly in a saline environment salt enters the plant and accumulates and can reach toxic concentrations.5
Salinity is the presence of high level of soluble salt in soil or water. Under these stress conditions there is a decrease in water uptake both during imbibitions and seedling establishment in the case of salt stress this can be followed by uptake of ions.6 By altering its metabolism, growth and development, abiotic stresses can directly or indirectly affect the physiological status of an organism and adversely affect agricultural productivity.7 The response of plants to saline stress is complex since it involves change in their morphology, physiology and metabolism and may be expected to vary in different growth stages of plant species. Furthermore these responses also may vary in different cultivars of the same plant. For example, Essa8 reported that germination percentage of soyabean (Glycine max) (L.) Merr.) was significantly reduced with increasing salinity levels.
Salinity decreases crop productivity and threatens the global food balance. It is present in soluble form from low level to high level in soil atmosphere and resulted in decreased water uptake both during imbibition and seedling establishment followed by uptake of ions.9 Germination and seedling development is very important for early establishment of plants under stress condition.10, 11 Selecting cultivars for rapid and uniform germination under salt stress conditions can contribute towards early seedling establishment. In view of the above, in the present study, effects of salt (NaCl) stress on the seed germination and seedling growth of Macrotyloma uniflorum and Vigna mungo was carried out with the following objectives:
i. To identify the physiological and morphological responses of selected leguminous crops and ii. To analyze the sensitivity and stress tolerance in selected crops.
Materials and Methods
Germination Experiment
This experiment was conducted during the year 2015 in the glass house of Department of Botany, DSB Campus, Kumaun University, Nainital. Seeds of selected crops (Vigna mungo and Macrotyloma uniflorum) were obtained from the healthy plants. Healthy, uniform seeds of selected crops were surface sterilized and washed with distill water and kept under six salt stress levels (0, 4, 8, 12, 16, 20 dsm-1) prepared using NaCl and referred as C, S1, S2, S3, S4 and S5, respectively. The seeds were placed in sterile petri dishes (9cm diameter/lined with two sterile filter paper with 5ml of distilled water or the respective salt solution). The petridishes were arranged in a complete randomized block design with 10 seeds per petridish and three replicate per treatment. Germination test were conducted under condition of 12h light/dark cycle with 14⁰C minimum and 24⁰C maximum temperature. A seed was considered germinated when radicle was 2mm long. The root and shoot length were measured on the 10th day. Shoot and root dry weight were recorded after oven drying at 60⁰C for 48 h.
Data analysis
Percent seed germination
After final count the germination percentage was calculated by the following formula12:
GP% = Number of total germinated seeds/ Total number of seeds tested x 100
Weight reduction percentage
The shoots and roots were separated and the dry weights were measured after oven drying at 60°C for 24 hours. According to each salt treatment, the dry weights, referred to the controlled, were calculated in percent by the following equations:
Dry weight (DW) percentage reduction
DWPR % = [1 – (dry weight salt stress / dry weight control)] x 100
Salt Tolerance Index (STI):
It is quantified by the ratio of the total dry weight in salt stress and control, and calculated by the following equation:
STI = (Total DW salt stress / Total DW control) x 100
Seed vigor index (SVI):
This index was determined by following Abdul and Anderson13:
Seed vigor index 1 = germination percentage × seedling length (root + shoot)/ 100
Seed vigor index 2 = germination percentage × seedling dry mass (root + shoot)/ 100
Statistical Analysis
The replicates were analyzed for the mean and standard error, while ANOVA using SPSS 16.0 software was done to prove the statistical significance of the results.
Results and Discussion
Analysis of variance showed significant effect on germination and seedling growth due to species as well as salt stress levels. Statistical analysis indicated that differences among species were significant (P>0.05) for germination percentage only while salt stress treatment showed significant (P>0.05) effect on all growth parameters except germination percentage (Table 1).
Table 1: Analysis of Variance (ANOVA) for traits investigated for the selected leguminous crop in response to salinity stress.
| Parameters | Mean Square | |||||
| Df | SL (cm) | RL (cm) | GP % | SDW (g) | RDW (g) | |
| Selected crops (M,V) | 1 | 0 .33ns | 0 .01ns | 64.0* | 0 .011ns | 0 .010ns |
| Salt Stress levels | 5 | 24.15* | 0 .009* | 5.91ns | 0.087* | 0.009* |
* Significant at 5%, and ns: not significant. SL: shoot length, RL: root length, GP%: germination percentage, SDW: shoot dry weight, RDW: root dry weight
Effect on germination percentage
In this experiment, highest seed germination was observed in V. mungo as compared to M. uniflorum at all salinity stress levels. In V. mungo, seed germination decreased with increasing salt stress level while in M. uniflorum, seed germination decreased upto S3 level and again increased in S4 and S5 levels (Fig. 1). In the present study, M. uniflorum showed higher germination percentage as compared to V. mungo (Table 2). Craig et al14 also reported that species vary widely in their ability to withstand salt stress. However, in both the species germination decreased with increasing salinity stress (Table 2). According to Naseri et al. 15 salinity reduces the water potential due to the effect of specific ions and prevents the absorption of water by seeds thus, resulting in decreased germination. Salinity reduces the ability of plants to utilize water and cause a reduction in growth rate, as well as changes in plant metabolic processes.16 Furthermore, it decreases plant growth and yield depending on the plant species and salinity levels.17
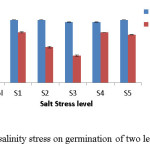 |
Figure 1: Effect of salinity stress on germination of two leguminous crops. Click here to View figure |
Effects on Seedling Growth
In the present study, seedling growth decreased with increasing salt stress level (Fig. 2).
Effects on Seedling Length
In both the crops, shoot length decreased with increasing salinity levels (Fig 3). At control V. mungo showed higher shoot length (8.5cm) as compared to M. uniflorum (7.3 cm). However, V. mungo showed higher susceptibility as compared to M. uniflorum. V. mungo produced higher (6.8 cm) root length while M. uniflorum produced lower (3.3 cm) root length at control while at highest (20dsm-1) salinity stress level root length was greater for M. uniflorum (Fig 4).
 |
Figure 2: Effect of salinity stress on seedling growth in two leguminous crops. Click here to View figure |
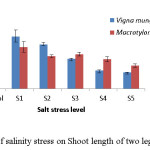 |
Figure 3: Effect of salinity stress on Shoot length of two leguminous crops. Click here to View figure |
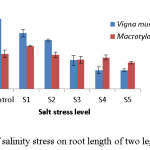 |
Figure 4: Effect of salinity stress on root length of two leguminous crops. Click here to View figure |
The root and shoot length are important parameters in plants because roots have direct contact with soil and absorb water from soil while shoot conduct it to the parts of the plant. Therefore, root and shoot length is a good indicator to analyse responses of plants to salinity stress.18 In the present study, a decrease was recorded with the increase of salt concentrations for both root and shoot length. The maximum length value was expressed in the control conditions and the minimum with the highest NaCl concentration (Table 2). According to Sreemvasulu19 the adverse effects of high salt concentration (salt stress) on plant growth are due to reduction in the osmotic potential of the soil solution that reduce plant available water and thus creating a water stress in plants or due to increase in the concentration of certain ions particularly Na+ which causes severe ion toxicity. Reduction in seedling height is common phenomenon of many crop plant grown under saline condition.20 Shoot and root length provides an important clue to the response of plants to salt stress.21 Naseri et al. 15 reported that with increasing salinity, root length was more affected than shoot length. Reduction in seedling growth as a result of salt stress has been reported in several others species.22
Effects on seedling dry weight
Shoot dry weight was inversely related to salinity stress levels and was relatively less sensitive as compared to root dry weight especially at higher salt concentration. V. mungo showed higher dry weight towards low salinity levels while M. uniflorum showed higher dry weight towards higher salinity levels (Fig 5).
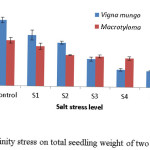 |
Figure 5: Effect of salinity stress on total seedling weight of two leguminous crops. |
The dry mass of seedlings grown in salt solutions also showed decline, indicating that the salt stress not only affected germination but also the growth of seedlings, which indicates that synthetic ability of seed, and thus, dry matter production of the seedlings, was affected. This is in conformity with the findings of Hakim et al. 23 Though V. mungo showed higher values for seedling dry mass the dry weight percentage reduction was higher in this species as compared to M. uniflorum.
Table 2: Means (± standard error) comparison of selected crops, salinity stress level and their interaction on the studied traits in germination experiment.
| SL (cm) | RL (cm) | SDW (g) | RDW ( g) | GP (%) | SV | |
| Species | ||||||
| Vigna mungo | 4.74±1.041 | 3.87±0.837 | 0.276±0.054 | 0.17±0 .019 | 99.0±0.89 | 8.55±1.898 |
| Macrotyloma uniflorum | 4.89±0.648 | 3.32±0.220 | 0.26±0.046 | 0.14±0 .014 | 72.22±11.013 | 6.05±1.283 |
| TREATMENT (dsm-1) | ||||||
| 0 | 7.90±.625 | 5.06±1.700 | 0.45±0.025 | 0.22±0.020 | 98.33±1.36 | 12.78±2.50 |
| 4 | 5.83±.640 | 4.75±.630 | 0.32±0.025 | 0.18±0.020 | 90±8.17 | 9.64±2.20 |
| 8 | 4.76±.735 | 4.00±.700 | 0.26±0.045 | 0.17±0.025 | 88.5±6.94 | 7.17±3.03 |
| 12 | 4.00±.290 | 2.79±.025 | 0.22±0.020 | 0.14±0.030 | 88.33±9.53 | 4.65±1.60 |
| 16 | 2.96±.700 | 2.42±.610 | 0.15±0.015 | 0.13±0.005 | 78.33±17.71 | 4.64±.710 |
| 20 | 2.02±0 | 1.80±0 | 0.12±00 | 0.11±00 | 70.16±21.93 | 3.81±0 |
Effects on initiation and completion of germination
The emergence time taken by V. mungo was 2 to 3 days while M. uniflorum took 3 to 4 days to initiation while completion time ranged from 6 to 10 days for V. mungo and 8-10 days for M. uniflorum (Table 3).
Table 3: Effect of salinity stress on initiation and completion of seed germination of selected leguminous crops (I = initiation in days, C = completion days).
| Salinity Level(dsm-1) | Selected leguminous crops | |||
| Vigna mungo | Macrotyloma uniflorum | |||
| I | C | I | C | |
| 0 | 2 | 6 | 3 | 8 |
| 4 | 2 | 6 | 3 | 8 |
| 8 | 2 | 10 | 3 | 10 |
| 12 | 2 | 10 | 4 | 10 |
| 16 | 3 | 10 | 4 | 10 |
| 20 | 3 | 10 | 4 | 10 |
Effect on seed vigor
Seed vigor index declined with the increase in salt concentrations. The maximum seed vigor index in terms of seedling length was recorded at controlled (15.29) condition and the minimum seed vigor index (3.05) were recorded at (12dsm-1) salinity level (Table 4). Seed vigor index declined with the increase in salt concentrations. Similar results were also observed by Cokkizgin24 for Phaseolus vulgaris. Seedling vigor index of maize was also significantly affected under different salt stresses.25, 26
Effects on Salt Tolerance Index
Salt tolerance index increased with increasing salt stress. V. mungo (263.22%) showed highest salt tolerance index as compared to M. uniflorum (235.35%). At 4 dsm– 1salinity stress both the selected leguminous crops showed the lowest salt tolerance index (Table 4). Vibhuti et al4 also reported that salt tolerance index decreased with the increase in salt stress and at 16 dsm-1 salinity stress all varieties showed the lowest salt tolerance.
Table 4: Effect of salinity on selected parameters of two leguminous crops.
| Selected leguminous crops / salt stress levels | DWPR% | SV1% | SV2% | STI% |
|
0dsm-1 |
||||
| Vigna mungo | 00 | 15.29 | 0.72 | 00 |
| Macrotyloma uniflorum | 00 | 10.28 | 0.59 | 00 |
|
4dsm-1 |
||||
| Vigna mungo | 26.38 | 11.85 | 0.53 | 121.07 |
| Macrotyloma uniflorum | 27.41 | 7.44 | 0.36 | 158.84 |
|
8dsm-1 |
||||
| Vigna mungo | 31.94 | 10.20 | 0.49 | 163.19 |
| Macrotyloma uniflorum | 43.54 | 4.14 | 0.19 | 182.41 |
|
12dsm-1 |
||||
| Vigna mungo | 43.05 | 6.25 | 0.39 | 242.15 |
| Macrotyloma uniflorum | 50.00 | 3.05 | 0.13 | 194.16 |
|
16dsm-1 |
||||
| Vigna mungo | 63.88 | 3.93 | 0.25 | 263.22 |
| Macrotyloma uniflorum | 53.22 | 5.35 | 0.23 | 235.35 |
|
20dsm-1 |
||||
| Vigna mungo | 69.44 | 3.81 | 0.22 | – |
| Macrotyloma uniflorum | 64.51 | 4.15 | 0.16 | – |
DWPR% = Dry weight percent reduction, SV = Seed vigor (1 for seedling length and 2 for seedling drymass), STI = Salt tolerance index
The accumulation of plant biomass is closely related to their productivity and varies considerably depending on the salinity and salt tolerance of variety.27,28 Salinity stress at vegetative stage of rice initiates significant alterations to many physiological processes of plant cells, including ion homeostasis, which all may cause to yield reduction.29
Conclusion
Identification and use of salt resistant crops is one of the strategies to reduce the negative effects of salinity on germination and plant growth. In the present study, all the studied traits were negatively affected with increasing salinity level. The difference in responses between species can be used to assess salt stress tolerance potential and salt tolerance index can be used to select salt tolerant species. At each salinity level, M. uniflorum showed higher salt tolerance index as compared to V. mungo so it can be cultivated in fields with salty soil.
Acknowledgements
Financial support from UGC, New Delhi in the form of major research project is gratefully acknowledged.
References
- Shahi, C. Vibhuti, Bargali, K. and Bargali, S. S. Influence of seed size and salt stress on seed germination and seedling growth of wheat (Triticum aestivum). Indian J Agric Sci. 2015a;85(9):1134-1137.
- Bhattacharjee, S. Triadimefon pretreatment protects newly assembled membrane system and causes up-regulation of stress proteins in salinity stressed Amaranthus lividus L. during early germination. J Environ Biol. 2008;29:805-810.
- Mahmoud, A. Germination of Cassia italica from Saudi Arabia. Arab Gulf J. Sci. Res. 1985;3:437-447.
- Vibhuti, Shahi, C. Bargali, K. and Bargali, S. S. Assessment of salt stress tolerance in three varieties of rice (Oryza sativa L.) J Prog Agric. 2015a;6(1):50-56.
- Arnold, P. How does salt water affect on plant growth (2011) (http://www.brighthub.com/environment/science-environmental/articles/89127.aspx).
- Prisco, J.T. and O`leary, J.W. Osmotic and “toxic” effects of salinity on germination of Phaseolus vulgaris L. Seeds. Turriaiba. 1970;20:177-184.
- Padalia, K. Bargali, K. Bargali, S.S. Present scenario of agriculture and its allied occupation in a typical hill village of central Himalaya, India. Indian J Agric Sci. 2017;87(1):132–141.
- Essa, T.A. Effect of salinity stress on growth and nutrient composition of three soyabean (Glycine max L. Merrill) cultivars. J Agric Crop Sci. 2002;188(2):86-93.
CrossRef - Yokoi, S. Quintero, F. J. Cubero, B. Ruiz, M.T. Bressan, R. A. Hasegawa, P. M. and Pardo, J. M. Differential expression and function of Arabidopsis thaliana NHX Na+/H+ antiporters in the salt stress response. Plant J. 2002;30:529–539.
CrossRef - Bargali, K. and Bargali, S. S. Germination capacity of seeds of leguminous plants under water deficit conditions: implication for restoration of degraded lands in Kumaun Himalaya. Trop Ecol. 2016;57(3):445-444.
- Awasthi, P. Karki, H. Vibhuti, Bargali, K. and Bargali, S.S. Germination and Seedling Growth of Pulse Crop (Vigna sp.) as affected by Soil Salt Stress. Curr Agric Res J. 2016;4(1):159-170.
CrossRef - Raun, S. Xue Q. Thlkowska K. Effect of seed priming on germination and health of rice (Oryza sativa L) seeds. Seed Sci Technol. 2002;30:451–8.
- Abdul, B.A.A. and Anderson, J.D. Viability and leaching of sugars from germinating barley. Crop Sci. 1970;10:31-34.
CrossRef - Craig, G.F. Bell, D.T. and Atkins, C.A. Responses to salt stress and water lodgings stress ten taxa of Acacia selected from naturally Saline areas of Western Australia. Australian J Bot. 1990;38:619-630.
CrossRef - Naseri, R. Mirzaci, A. Emami, T. and Vafa, P. Effect of salinity on germination stage of rapeseed cultivars (Brassica napus L.). Int J Agric Crop Sci. 2012;4(13):918-922.
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002;25:239-250.
CrossRef - Yadav, S. S. McNeil, D. L. Redden, R. and Patil, S. A. Climate change and management of cool season grain legume crops. Springer, Dordrecht; Heidelberg. 2010;460.
CrossRef - Baghbani, A. Forghani, A.H. and Kadkhodaie, A. Study of salinity stress on germination and seedling growth in greenhouse cucumber cultivars. Int J Basic App Sci Res. 2013;3(3):1137-1140.
- Sreenivasulu, N. Sopory, S. K. and Kishor, P. B. Deciphering the regulatory mechanisms of abiotic stress tolerance in plants by genomic approaches. Gene. 2007;388(1-2):1-13.
CrossRef - Vibhuti, Shahi, C. Bargali, K. and Bargali, S. S. Seed germination and seedling growth parameters of rice (Oryza sativa L.) varieties as affected by salt and water stress. Indian J Agric Sci. 2015b;85:102-108.
- Shahi, C. Vibhuti, Bargali, K. and Bargali, S. S. How Seed Size and Water stress affect the seed germination and seedling growth in wheat varieties? Curr Agric Res J. 2015b;3(1):60-68.
CrossRef - Akram M, Ashraf M Y, Ahmad R, Waraich E A, Iqbal J and Mohsan M.. creening for salt tolerance in maize (Zea mays L.) hybrids at an early stage. Pakistan J Bot. 2010;42:141–51.
- Hakim, M. A. Bengum, A.S. J. Hanafi, M. M. Ismail, M. R. and Selamat, A. Effect of salt stress on germination and early seedling growth of rice. Afr J Biotechnol. 2010;9(13):1911-1918.
CrossRef - Cokkizgin, A. Salinity stress in common bean (Phaseolus vulgaris L.) seed germination. Notulae Botanicae Horti Agrobotanici. 2012;40(1):177-182
- Janmohammadi, M. Dezfuli, P. M. and Sharifzadeh, F. Seed invigoration techniques to improve germination and early growth of inbred line of maize under salinity and drought stress. Gen App Plan. Physiol. 2008;34:215-226.
- Pantola, S. Vibhuti, Bargali, K. and Bargali, S. S. Screening of three leguminous crops for drought stress tolerance at germination and seedling growth stage. Indian J Agric Sci. 2017;87(4):467–72.
- Ali, M.N. Yeasmin, L. Gantait, S. Goswami R. and Chakraborty, S. Screening of rice landraces for salinity tolerance at seedling stage through morphological and molecular markers. Physiol. Mol. Biol. Plants. 2014;20:411-423.
CrossRef - Usatov, A.V. Klimenko, A.I. Azarin, K.V. Gorbachenko O.F. and Markin N.V. DNA-markers of sunflower resistance to the downy mildew (Plasmopara halstedii). Am. J Biochem Biotech. 2014;10:125-129.
CrossRef - Kirill V. A, Andrey, Alabushev, V. Usatov, A.V. Kostylev, P.I. Kolokolova, N.S. and Usatova O. A. Effects of Salt Stress on Ion Balance at Vegetative Stage in Rice (Oryza sativa L.). J Biol Sci. 2016;16(1):76.81.

