Introduction
India is agriculture- based country and is the second largest producers of onion in the world. Madhya Pradesh is one of the major onions producing States. In today’s scenario stress is a matter of concern for all living beings including plants. Sudden change in environment cause stress which affects photosynthesis.1 Yield is dependent on rate of photosynthesis. Change in temperature and water availability can decrease plant growth by affecting various physiological processes like photosynthesis, water balance and antioxidative defence mechanism.2 Water stress cause decrease in chlorophyll content in Triticum durum seed.3
With the progression of world food demand it is necessary to increase tolerance of crops to environmental stress to maintain adequate food supplies.The predominant light absorbing pigment in photosynthesis is chlorophyll which is also required to maintain stability of light harvesting complex proteins.4 Decrease in chlorophyll content thus would adversely affect photosynthesis. Inhibition of chlorophyll biosynthesis leads to accumulation of tetrapyrrole intermediates which when illuminated can result in generation of reactive oxygen species (ROS).5,6 As chloroplast is the centre for high energetic reaction of photosynthesis along with abundant oxygen supply, it is major site for generation of reactive oxygen species.7 Rate of photosynthesis can be determined using chlorophyll content.8 Physiological character such as chlorophyll can help to assess effect of drought condition on growth and production as it is directly related to rate of photosynthesis.9 Stress lead to production of reactive oxygen species resulting in degradation of chlorophyll and peroxidation of lipid membrane.10
Silicon has been found to alleviate the negative effect of stress by restoring normal growth and physiological processes in plants. In sorghum grown in drought condition, silicon application improved the rate of photosynthesis.11 In a study on capsicum silicon alleviated water deficit stress induced damage in photosynthetic mechanism and improved photosynthesis.12 Extent of photosynthesis inhibition is inversely related to ratio of chlorophyll a/b.13 Silicon is believed to increase chlorophyll content and chlorophyll a/b ratio under stress condition.14 Plants exposed to different environmental stresses including disease infections exhibit changes in membrane permeability that lead to loss of membrane integrity.15 Membrane lipid peroxidation is measured in terms of malondialdehyde, an indicator of oxidative damage.16
Therefore the present study was aimed at evaluating effect of environmental stress on chlorophyll content of onions and the use of soluble silica for its remedy.
Materials and Methods
A field study was conducted on onion crop variety Allora from January to May 2018 at Loharpipliya village, Dewas road. Geographical location of fields selected as per Latitude
Longitude read by GPS 76 Garmin was N- 22 54’40″ –41.4″ E- 075 59’ 35.1″– 37″. Seeds of onion were first used to form ropa [small plant] which were then further transferred to get onion crop. Randomized block design was used with 2.5 m2 area for each block. Each block contains approx. 250-260 plants. Soluble silica as AgriboosterTM was used in liquid form in eight treatments as follows:
T1- without fertilizer and soluble silica (Control)
T2, T3 T4- foliar spray of soluble silica viz, 7.5, 10 and 12.5 ml/ lit respectively
T5- only given N, P, K fertilizer
T6, T7, T8- fertilizer + foliar spray of soluble silica viz, 7.5, 10 and 12.5 ml/ lit respectively
After one month of sowing “ropa”, three sprays with soluble silica were given at interval of 15 days. Leaf samples were collected after 15 days of each treatment and analysed for following contents in triplicates:
Determination of Chlorophyll a, b and carotenoid content: Chlorophyll was extracted from leaves on the basis of its solubility using 80% acetone. Chlorophyll a, b and carotenoid were calculated using absorbance of supernatant at 470, 646 and 663 in below equation17
Chlorophyll a (mg/g) = 12.21(A663) – 2.81 (A646)
Chlorophyll b (mg/g) = 20.13 (A646) – 5.03 (A663)
Carotenoid (mg/g) = [1000(A470)-3.27 (Chl a)- 104 (Chl b)]/229
Determination of Malondialdehyde(MDA) content–
It was extracted from leaves using 5% trichloroacetic acid and then estimated by reacting with thiobarbituric acid prepared in 20% trichloroacetic acid. The absorbance of mixture after boiling for 30 minutes and centrifugation was taken at 532 nm.18 Concentration of MDA was calculated using extinction coefficient (ε= 155 mM-1cm-1)
Chlorophyll a/b ratio–
It was calculated by dividing chlorophyll a content by Chlorophyll b content.
Result and Discussion
Results are expressed as mean ± SD. p value was calculated to test significant difference following the protocols of Ashfaq et al., 2019; David et al., 2019; Ullah et al., 2019.19-21 Anova was applied to test significant difference within the treatments.
Table 1: Effect of silica on Malondialdehyde, chlorophyll and carotenoid content in leaves of onion after 15 days of first silica spray
| Treatments | MDA (µmoles/g) | Chlorophyll a (mg/g) | Chlorophyll b (mg/g) | Chlorophyll a/b ratio | Cartenoid (mg/g) |
| T1 | 0.022 ± 0.001 | 8.86 ± 0.91 | 7.34 ± 0.38 | 1.2 ± 0.06 | 0.022 0.002 |
| T2 | 0.014 ± 0.006ans,bns | 9.41 ± 0.88ans, bns | 7.47 ± 0.65ans, bns | 1.25 ± 0.02ans, bns | 0.018 ± 0.014 ans, bns |
| T3 | 0.013 ± 0.003a**,b* | 10.33 ± 0.37ans, bns | 8.02 ± 0.73ans, bns | 1.28 ± 0.07ans, bns | 0.021 ± 0.003 ans, bns |
| T4 | 0.018 ± 0.001a*, bns | 10.28 ± 0.93ans, bns | 8.12 ± 0.45ans, bns | 1.26 ± 0.17ans, bns | 0.019 ± 0.016ans, bns |
| T5 | 0.02 ± 0.006ans, bns | 9.33 ± 1.21ans | 8.69 ± 1.14ans | 1.09 ± 0.3ans | 0.026 ± 0.01 ans |
| T6 | 0.01 ± 0.006ans,b* | 12.17 ± 1.01 a*,b* | 10.31 ± 1.32 a*,bns | 1.18 ± 0.05ans, bns | 0.015 ± 0.007 ans, bns |
| T7 | 0.01 ± 0.003a*,b* | 12.21 ± 0.94 a*,b* | 8.04 ± 2.79ans, bns | 1.6 ± 0.39ans, bns | 0.018 ± 0.004 ans, bns |
| T8 | 0.013 ± 0.0005a***,b** | 12.84 ± 1.36 a*,b* | 9.98 ± 1.27 a*,b ns | 1.28 ± 0.03ans, bns | 0.018 ± 0.007 ans, bns |
Table 2: Effect of silica on Malondialdehyde, chlorophyll and carotenoid content in leaves of onion after 15 days of second silica spray.
| Treatments | MDA (µmoles/g) | Chlorophyll a (mg/g) | Chlorophyll b (mg/g) | Chlorophyll a/b ratio | Cartenoid (mg/g) |
| T1 | 0.017 ± 0.003ans, bns | 7.29 ±1.12 | 4.11 ±2.89 | 1.67 ±0.61 | 0.015 ± 0.006 |
| T2 | 0.012 ± 0.0005ans, bns | 9.46 ± 0.89 a*,b ns | 6.54 ± 0.71a ns, b* | 1.44 ± 0.04ans, bns | 0.017 ± 0.003 a ns, b* |
| T3 | 0.012 ± 0.003ans, bns | 11.77 ±2.27 a*,b* | 8.45 ±3.07ans, bns | 1.44 ± 0.23ans, bns | 0.025 ± 0.009 ans, bns |
| T4 | 0.011 ± 0.002 ans, bns | 10.85 ± 0.37 a***,bns | 7.52 ±0.46ans, bns | 1.44 ± 0.03 a ns, b* | 0.015 ± 0.008 ans, bns |
| T5 | 0.015 ± 0.002ans | 9.87 ±0.73 a* | 7.78 ± 0.22ans, bns | 1.26 ± 0.08ans | 0.027 ± 0.004 ans |
| T6 | 0.006 ± 0.003a*,b* | 12.08 ± 1.42 a**,bns | 9.27 ± 1.83 a*,b ns | 1.32 ± 0.22ans, bns | 0.020 ± 0.01 ans, bns |
| T7 | 0.010 ±0.003 a*,b ns | 10.98 ± 0.94 a*,b ns | 9.13 ±1.6 a*bns | 1.21 ± 0.13ans, bns | 0.021 ± 0.008 ans, bns |
| T8 | 0.006 ± 0.003 a***,b*** | 12.32 ± 0.9 a**,b* | 8.63 ±1.03 a*, bns | 1.44 ± 0.28ans, bns | 0.017 ± 0.003 a ns, b* |
Table 3: Effect of silica on Malondialdehyde, chlorophyll and carotenoid content in leaves of onion after 15 days of third silica spray.
| Treatments | MDA (µmoles/g) | Chlorophyll a (mg/g) | Chlorophyll b (mg/g) | Chlorophyll a/b ratio | Cartenoid (mg/g) |
| T1 | 0.042± 0.01 | 11.02 ± 1.66 | 8.52 ± 1.40 | 1.32 ± 0.30 | 0.03 ± 0.01 |
| T2 | 0.013 ± 0.003a*,b* | 15.72 ±0.63a*, b*** | 10.82± 0.59 ans,bns | 1.44± 0.13 ans,b* | 0.01 ± 0.005 ans, bns |
| T3 | 0.016 ± 0.002ans, b* | 14.32 ± 0.61a*,b** | 10.30± 0.50ans, bns | 1.38± 0.04 ans, b** | 0.01 ± 0.003 ans, bns |
| T4 | 0.014 ± 0.002ans, b*** | 15.55 ± 0.56a*,b*** | 11.44± 0.57 a*,b* | 1.08 ± 0.06ans, bns | 0.007 ± 0.004 ans,b* |
| T5 | 0.022 ± 0.001ans | 11.46 ± 0.40a ns | 9.81 ± 0.65ans | 1.16 ± 0.05ans | 0.01 ± 0.003 ans |
| T6 | 0.017 ± 0.001ans,b* | 14.22 ± 0.15 a*,b*** | 11.12± 0.30 a*,b* | 1.27± 0.04 ans, bns | 0.009 ± 0.003 ans,b* |
| T7 | 0.018 ± 0.001 a*,b* | 14.18 ± 0.61 a*, b*** | 9.63 ± 1.34 ans, bns | 1.37 ± 0.05ans, b** | 0.01 ± 0.08 ans, bns |
| T8 | 0.011 ± 0.005 a***, b*** | 14.11 ± 1.07 a*,b* | 11.75 ± 2.10 ans, bns | 1.27± 0.08 ans, bns | 0.01 ± 0.01 ans, bns |
a indicates p value compared to T1 and b indicates p value compared to T5
*indicates p value < 0.05 and is significant as compared to control
**indicates p value < 0.01 and is highly significant as compared to control
***indicates p value < 0.001 and is extremely significant as compared to control
Table 4: showing F value between different treatments
|
Parameters |
F value between T2,T3 and T4 after first treatment | F value between T6,T7 and T8 after first treatment | F value between T2,T3 and T4 after second treatment | F value between T6,T7 and T8 after third treatment | F value between T2,T3 and T4 after third treatment | F value between T6,T7 and T8 after third treatment |
| Chlorophyll a | 1.83 | 1.33 | 2.19 | 1.42 | 1.99 | 0.083 |
| Chlorophyll b | 1.12 | 1.41 | 1.17 | 0.16 | 3.69 | 2.01 |
| Chlorophyll a/b | 0.12 | 3.72 | 0.25 | 0.921 | 1.42 | 3.33 |
| carotenoid | 0.057 | 0.056 | 1.11 | 0.875 | 4 | 0.916 |
| MDA | 1.25 | 0.63 | 0.94 | 0.44 | 1 |
3.54 |
Tabulated F value at 2, 7 degree of freedom is 4.74. All the calculated values are less than tabulated value therefore difference is insignificant.
Table 5: showing correlation between parameters
| First silica spray | Second silica spray | Third silica spray | ||||||||||||||
| Chl a | Chl b | Chl a/b | Carotenoid | MDA | Chl a | Chl b | Chl a/b | Carotenoid | MDA | Chl a | Chl b | Chl a/b | Carotenoid | MDA | ||
| First silica spray | Chl a | 1 | 0.6 | 0.34 | -0.18 | -0.35 | 0.62 | 0.42 | -0.4 | 0.08 | -0.58 | 0.25 | 0.44 | -0.13 | -0.43 | -0.44 |
| Chl b | 1 | -0.53 | -0.43 | -0.1 | 0.37 | 0.178 | 0.02 | 0.35 | -0.48 | 0.04 | 0.3 | -0.27 | -0.26 | -0.25 | ||
| Chl a/b | 1 | 0.29 | -0.21 | 0.2 | 0.27 | -0.13 | -0.27 | 0.03 | 0.18 | 0.08 | 0.15 | -0.14 | -0.16 | |||
| Carotenoid | 1 | 0.18 | -0.04 | 0.08 | -0.1 | -0.17 | 0.39 | -0.28 | -0.31 | 0.13 | 0.23 | 0.11 | ||||
| MDA | 1 | -0.43 | -0.1 | 0.1 | 0.34 | 0.57 | -0.58 | -0.39 | -0.28 | 0.31 | 0.4 | |||||
| Second silica spray | Chl a | 1 | 0.81 | -0.27 | 0.09 | -0.43 | 0.39 | 0.59 | -0.11 | -0.5 | -0.67 | |||||
| Chl b | 1 | -0.72 | 0.1 | -0.42 | 0.2 | 0.2 | 0.03 | -0.22 | -0.57 | |||||||
| Chl a/b | 1 | -0.2 | 0.21 | -0.04 | 0.17 | -0.28 | -0.17 | 0.36 | ||||||||
| Carotenoid | 1 | 0.03 | -0.09 | -0.2 | 0.11 | 0.08 | 0.01 | |||||||||
| MDA | 1 | -0.57 | -0.47 | -0.11 | 0.03 | 0.45 | ||||||||||
| Third silica sprayt | Chl a | 1 | 0.59 | 0.21 | -0.58 | -0.51 | ||||||||||
| Chl b | 1 | -0.42 | -0.91 | -0.5 | ||||||||||||
| Chl a/b | 1 | 0.48 | 0.11 | |||||||||||||
| Carotenoid | 1 | 0.45 | ||||||||||||||
| MDA | 1 | |||||||||||||||
 |
Photograph 1: Showing treatment of onion with soluble silica Click here to View Photograph |
 |
Photograph 2: Showing onion leaves after third silica spray Click here to View Photograph |
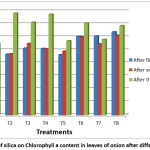 |
Figure 1: Effect of silica on Chlorophyll a content in leaves of onion after different treatments Click here to View Figure |
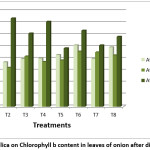 |
Figure 2: Effect of silica on Chlorophyll b content in leaves of onion after different treatments Click here to View Figure |
Chlorophyll and Carotenoid Content
Chlorophyll content is influenced by various environmental factors like light intensity, temperature, water content etc. Some of the important role of carotenoids is in photosynthesis and photoprotection.22 In the present study Chlorophyll a content was found to be significantly increased in all soluble silica treated leaves of onion as compared to T1 (irrigated with only water) and T5 (supplied with routine fertilizer) in all the stages. Chlorophyll content in green leaves of susceptible varieties of durum wheat decreased under environmental stress.23 Under drought stress change in chlorophyll content occurs which depends upon plant species and conditions. In some plant decrease is observed in chlorophyll content and in some chlorophyll content increase under stress.24 All the three concentrations of silica viz, 7.5, 10, 12.5 ml/ lit have the same effect on chlorophyll and carotenoid content as calculated using ANOVA (Table 4).
Application of Silicon has shown to improve fresh and dry weight, chlorophyll concentration, rate of photosynthesis, conduction through mesophyll cells and water use efficiency under salinity stress.25,26 Supplementation with silica can maintain level of chlorophyll in capsicum under drought stress.12 Similar results were seen in drought stressed rice.27 Turan et al.,28 on P. vulgris and Taffouo et al.,29 on Vigna subterranean L observed decrease in chlorophyll content under plants exposed to salt stress. The reason for decrease in chlorophyll content under stress is attributed to both decreases in chlorophyll synthesis as well as increase in degradation of chlorophyll by chlorophyllase enzyme.30 Si induces synthesis of new chlorophylls and also protects existing chlorophyll from oxidative stress induced by salinity.31 Supplementation with silica increased chlorophyll a, b and carotenoid concentration in control and salt stressed in tobacco leaves.32 Tuna et al.,33 reported that silicon treated wheat plants have higher concentration of pigments. As shown in table 5, high positive correlation was observed between Chlorophyll a and chlorophyll b after each treatment which indicates that chlorophyll b concentration is affected by chlorophyll a in leaves.
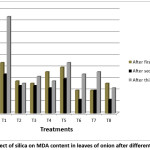 |
Figure 3: Effect of silica on MDA content in leaves of onion after different treatments Click here to View Figure |
Oxidative Stress
In open field, environmental conditions are not constant. Environmental changes disturb the control system of plants due to accumulation of reactive oxygen species which cause damage to plants and affects its growth.34 With application of soluble silica in absence of fertilizer, MDA content decreased significantly as compared to T1 (only water) and T5 (only fertilizer). Decrease in lipid peroxidation measured by MDA content indicates increased tolerance of plant to changing environmental condition. In silica treated plants membrane lipid peroxidation is decreased indicating greater membrane stability.35 Treatment with soluble silica along with fertilizer also decreased MDA content significantly as compared to T1 and T5 indicating more tolerance in plants. MDA content was increased under saline stress as observed in alfaalfa cultivators by Babakhani et al.,36 Wang et al.,37 reported increased in MDA concentration in sensitive variety of alfalfa under salinity stress. Malondialdehyde content was decreased in presence of soluble silica.38 Potassium silicate ameliorated the effect of infectious disease in onion indicated by decrease in lipid peroxidation and increasing membrane stability.39 Alleviation of lipid peroxidation by supplementation of silicon was observed in F. oxysporumf. sp. cubense, cotton- Ramularia areola, rice- P. oryzae, sorghum- C. sublineolum and wheat- P. oryzae marked by decrease amount of MDA.40,41
Pretreatment with silica before exposing plant to salt stress resulted in decrease in MDA content which shows increased tolerance of plant towards oxidative stress caused by salinity stress.42 Similar results were reported by Al‐aghabary et al.,43 In Boragoofficinalis L. MDA content was decreased in aluminium treated plant supplied with silicon.44 MDA content was found to be negatively correlated with both Chlorophyll a and Chlorophyll b which shows that under stress condition with increase in content of MDA, there occur decrease in pigment concentration (Table 5).
Conclusion
Decrease in productivity due to sudden environmental changes like adverse climatic changes, inadequate rainfall, abnormal soil properties etc. is a big challenge faced by farmers. Keeping in mind above problem the present study was designed to get a solution for it. Soluble silica according to the results proved to be helpful in reducing MDA concentration and elevating chlorophyll content. It can be concluded that application of soluble silica helps to enhances productivity by improving rate of photosynthesis. Farmers are suggested to use soluble silica to upgrade their yield and product quality.
Acknowledgements
We express our sincere thanks to Dr. Suresh T. Silawat, Principal, Govt. Holkar Science College,Indore (M.P.) for providing necessary laboratory facilities and encouragement.
Funding Sources
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of Interest
Authors declare no conflict of interest.
References
- Ashraf, M. and Harris, P.J.C.. Photosynthesis under stressful environments: an overview. Photosynthetica. 2013; 51: 163-190.
CrossRef - Hasannuzaman, M., Nahar, K., Fujita, M.,. Extreme temperature responses, oxidative stress and antioxidant defense in plants. In K Vahdati, C Leslie, editors. Abiotic stress- plant responses and applications in agriculture. Intech. Doi: 10.5772/54833.2013; 169–205.
- Ghozlene, I., Reda, D.M., Nedjoud, G., Houria, B., Ali.C.. Oxidative stress, chlorophyll content and ROS production and localization in Triticum durumseed. Annals of Biological Research.2013; 4 (5):11-15.
- Kuttkat, A., Edhofer, I., Eichaker, L.A., Paulsen, H.. Light-harvesting chlorophyll a/b-binding protein stably inserts into etioplast membranes supplemented with Zn-pheophytin a/b. J Biol Chem.1997; 272: 20451–20455.
CrossRef - Gupta, I. and Tripathy,B.C.. Oxidative stress in cucumber (Cucumissativus L) seedlings treated with acifluorfen. Ind J BiochemBiophys.2000; 37: 498–505.
- Vavilin, D.V. andVermaas,W.F.J..Regulation of the tetrapyrrole biosynthetic pathway leading to heme and chlorophyll in plants and cyanobacteria.Physiol Plant.2002;115: 9–24.
CrossRef - Asada,K..Production and Action of Active Oxygen Species in Photosynthetic Tissues. CRC Press, Florida. 1994; 77–104.
- Chaoyang, W., Zheng, N., QuanT.,Wenjiang. H..Estimating Chlorophyll Content from Hyperspectral VegetationIndices. Modeling and Validation Agricultural and Forest Meteorology.2008;148:1230-1241.
CrossRef - Li, R. H.., Pei-guo, G.U.O., Baum, M., Grando, S., Ceccarelli, S,. Evaluation of Chlorophyll Content and Fluorescence Parameters as Indicators of Drought Tolerance in Barley. Agricultural Sciences in China.2006; 5(10): 751–757.
CrossRef - Verma, S. and Mishra, S.N. Putrescine alleviation of growth in salt stressed Brassica juncea by inducing antioxidative defense system. Journal of plant physiology.2005; 162: 669-677.
CrossRef - Hattori, T., Inanga, S., Araki H., An, P., Morita, S., Luxova, M., Lux, A. Application of silicon enhanced drought tolerance in sorghum bicolour. Physiol Plant.2005; 123:459-466.
CrossRef - Lobato, A.K.S., Coimbra, G.K., Neto, M.A.M., Costa, R.C.L., Filho, B.G.S., Neto, C.F.O., Luz, L.M., Barreta, A.G.T., Pereira, B.W.F., Alves, G.A.R., Monteiro, B.S., Marochio, C.A. Protective action of silicon on water relations and photosynthetic pigments in pepper plants induced to water deficit. Res J Biol Sci.2009; 4: 617-623.
- Aro, E.M., McCaffery, S., Anderson, J.M. Photoinhibtion and D1 protein degradation in peas acclimated to different growth irradiances. Plant Physiol.1993; 103:835-843.
CrossRef - Powles, S.B..Photoinhibition of photosynthesis induced by visible light.Annu Rev Plant Physiol Plant Mol Bio.1984; 35:15-44.
CrossRef - Blokhina, O., E. Virolainen, K.V. Fagerstedt,. Antioxidants, oxidative damage and oxygendeprivation stress: a review, Annals of Botany.2003; 91: 179-194.
CrossRef - Del, R., Stewart, D., Pellegrini, N.,.A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress.Nutrition, Metabolism and Cardiovascular.2005; 15: 316-328.
CrossRef - Lichtenthaler, H. K. and Wellburn, A. R..Determinations oftotal carotenoids and chlorophylls a and b of leaf extracts indifferent solvents, Biochem. Soc. Trans.,1983;11, 591–592.
CrossRef - Heath,R.L.,and Packer, L.. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation.Arch BiochemBiophys.1968; 125: 180-198.
CrossRef - Ashfaq, S., Ahmad, M., Zafar, M., Sultana, S., Bahadur, S., Ullah, F., Zaman, W., Ahmed, S.N., Nazish, M., 2019. Foliar micromorphology of Convolvulaceous species with special emphasis on trichome diversity from the arid zone of Pakistan. Flora: Morphology, Distribution, Functional Ecology of Plants 255, 110-124.
CrossRef - David, M., Ain, Q.u., Ahmad, M., Zaman, W., Jahan, S., 2019. A biochemical and histological approach to study antifertility effects of methanol leaf extract of Asplenium dalhousiae Hook. in adult male rats. Andrologia 51, e13262.https://doi.org/10.1111/and.13262
CrossRef - Ullah, F., Nasar Shah, S., Zaman, W., Zafar, M., Ahmad, M., Ayaz, A., Sohail, A., Saqib, S., 2019. Using palynomorphological characteristics for the identification of species of Alsinoideae (Caryophyllaceae): a systematic approach. Grana 58, 174-184.
CrossRef - Xiao, X., Xu, X., Yang, F. Adaptive responses to progressive drought stress in two Populuscathayana populations. Silva Fennica.2008; 42(5): 705-719.
CrossRef - Khayatnezhad, M., Zaeifizadeh, M., Gholamin,R..Effect of end season drought stress on chlorophyll fluorescence and content of antioxidant enzyme superoxide dismutase (SOD) in susceptible and tolerant genotypes of durum wheat, African Journal of Agricultural Research.2011; 6(30): 6397-6406.
CrossRef - Sepehri, A. and Golparvar, A.R.,. The Effect of Drought Stress on Water Relations, Chlorophyll Content and Leaf Area in Canola Cultivars (Brassica napusL.). ElectronicJournal of Biology.2011;7(3): 49-53.
- Haghighi M, Pessarakli M.. Influence of silicon and nano–silicon on salinity tolerance of cherry tomatoes (Solanumlycopersicum L.) at early growth stage. ScientiaHorticulturae.2013; 161:111–117.
CrossRef - Pessarakli, M..Using Bermudagrass (Cynodondactylon L.)In Urban desert landscaping and as a forage crop for sustainable agriculture in arid regions and combating desertification. International Journal of Water Resources and Arid Environments. 2015;4(1):8–14.
- Chen, W., Yao, X.Q., Cai, K.Z., Chen, J. Silicon alleviates drought stress of rice plants by improving plant water status, photosynthesis and mineral nutrient absorption. Biol trace Elem Res.2011; 142: 67-76.
CrossRef - Turan, M.A., Turkmer, N., Taban, N.,.Effect of NaCl on stomatal resistance and proline, chlorophyll, NaCl and K concentrations of lentil plants.Journal of Agronomy.2007; 6: 378–381.
CrossRef - Taffouo, V.D.,Wamba, O.F., Yombi, E., Nono, G.V., Akoa, A.,. Growth, yield, water status and ionic distribution response of three bambara groundnut (Vigna subterranean (L.) verdc.) landraces grown under saline conditions. International Journal of Botany.2010; 6: 53–58.
CrossRef - Santos, C.V..Regulation of chlorophyll biosynthesis and degradation by salt stress in sunflower leaves.ScientiaHorticulturae.2004; 103: 93–99.
CrossRef - Shekari, F., Abbasi,A.,Mustafavi,S.H. . Effect of silicon and selenium on enzymatic changes and productivity of dill in saline condition. J. Saudi Soc. Agric. Sci.2017;16(4), 367-374.
CrossRef - Hajiboland, R. and Cheraghvareh, L..Influence of Si Supplementation on Growth and Some Physiological and Biochemical Parameters in Salt- Stressed Tobacco (Nicotianarustica L.) Plants Journal of Sciences, Islamic Republic of Iran.2014; 25(3): 205- 217.
- Tuna, A.L., Kaya, C., Higgs, D., Murillo-Amador, B., Aydemir, S., Girgin, A.R.,. Silicon improves salinity tolerance in wheat plants. Environ. Exp. Bot.2008; 62:10–16.
CrossRef - Parent, C., Capelli, N., Dat, J..Reactive oxygen species, stress and cell death in plants C. R. Biologies.2008; 331(4): 255-261.
CrossRef - Liang, Y.C., Liang, Y., Chen, Q., Liu, Q., Zhang, W., Ding, R..Exogenous silicon (Si) increases antioxidant enzymeactivity and reduces lipid peroxidation in roots of salt stressed barley (HordeumvulgareL.). J. Plant Physiol.2003; 160: 1157-1164.
CrossRef - Babakhani, B., Khavari–Nejad, R.A., Hassan SajediR.. Biochemical responses of Alfalfa (Medicagosativa L.) cultivars subjected to NaCl salinity stress. Afr J Biotechnol.2011; 10:11433–11441.
- Wang, W.B., Kim, Y.H., Lee,H.S.. Analysis of antioxidant enzyme activity during germination of alfalfa under salt and drought stresses. Plant PhysiolBiochem.2009;47:570–577.
CrossRef - Rangwala, T., Bafna, A., Gupta, R., Vyas, N. Role of soluble silica in alleviating oxidative stress in soybean crop. Indian J. Agric. Res.2018.;52 (1): 9-15.
CrossRef - Mamdouh, M. A. K., Nashwa, A.H. F., Medhat, S. A. M., Nabil I.E. -Effectiveness of Potassium Silicate in Suppression White Rot Disease and Enhancement Physiological Resistance of Onion Plants, and its Role on the Soil Microbial Community Middle East Journal of Agriculture Research.2017; 6 (2):01-19.
- Debona, D., Rodrigues,F.A., Rios, J.A.. The effect of silicon on antioxidant metabolism of wheat leaves infected by Pyriculariaoryzae. Plant Pathol.2014; 63: 581-589.
CrossRef - Domiciano, G.P., Cacique,I.S.,Freitas, C.C.. Alterations in gas exchange and oxidativemetabolism in rice leaves infected by Pyriculariaoryzae are attenuated by silicon.Phytopathology.2015; 105: 738-747.
CrossRef - Enteshari, S., Alishavandi, R., Delavar, K..Interactive effects of silicon and NaCl on some physiological and biochemical parameters in BoragoofficinalisL. Iranian Journal of Plant Physiology.2011; 2(1): 315- 320.
- Al‐aghabary, K., ZhujunZ., Qinhua, S. Influence of silicon supply on chlorophyll content, chlorophyll fluorescence, and antioxidative enzyme activities in tomato plants under salt stress. Journal of plantnutrition.2004;27: 2101–2115.
CrossRef - Shahnaz, G., Shekoofeh, E., Kourosh, D., Moohamadbagher, B..Interactive effects of silicon and aluminum on the malondialdehyde (MDA), proline, protein and phenolic compounds in Boragoofficinalis L. Journal of Medicinal Plants Research.2011; 5(24):5818-5827.


