Introduction
An estimated 3.3 million metric tons of pesticides are utilized annually having turnover about $40 billion. Numerous systemic NEOs, including imidacloprid and thiamethoxam, have proven successful in eliminating insects having piercing-sucking mouthpart, they are notoriously difficult to control 1. The Imidacloprid also known as “1-[(6-chloro-3-pyridinyl) methyl] -N-nitro-2-imidazolidinimine)”Chloropyridyl methyl (imidacloprid, thiacloprid, acetamiprid, and nitenpyram) and chlorothiazolyl methyl (thiamethoxam, and CLO) are the two new classes of neonicotinoid insecticides that were released in the market. The first Neo imidacloprid and thiamethoxam were approved for use in 1991 and 1998, respectively, and further Neo was released in the market between 1995 and 20022. Imidacloprid, the first neonicotinoid, is one of the insecticides with the quickest sales growth and has been applied to more than 140 crops in almost 120 nations. It is unlikely that any of the substances will dissipate into the atmosphere or water due to Henry’s law and the low volatility pressure (1.0 10-7 mmHg)3. A second-generation neonicotinoid pesticide is thiamethoxam. Because it binds to nicotinic acetylcholine receptors in the core nervous system. It is now one of the most efficient pesticides against aphids, whiteflies, and thrips4. By the application of lower dose, the first deposits of total imidacloprid residue in that was found in fresh and dried cardamom were about 1.91 and 7.23 µg g1, respectively. The initial residues in fresh and dried capsules by the application of higher dose were 3.94 and 14.72 µg g-1, respectively. After application of lower dose of 21 and 28 days, and after both the higher and lower doses combined for 28 days, the residues was not detected below the quantitation limit of 0.01 µg g-15.The dissipation dynamics of IMI in CPA followed first-order kinetics, with a half-life of 6.48-7.29 d, after application of IMI at a dosage of 467 mg a. i. L-1 for three times with an interval of 7 d. IMI made up the majority of CPA, with urea, guanidine, and olefin, 5-hydroxy, and 6-chloronicotinic acid making up minor portions6.The residues of b-cyfluthrin and imidacloprid in samples of brinjal were measured using “Gas Liquid Chromatography (GLC)” and “high performance liquid chromatography (HPLC)”, respectively, at 0, 1, 3, 5, 7, 10, and 15 days after the last treatment. The half-life periods for b-cyfluthrin were found to be 1.74 and 1.39 days at single and double the application rate, while for imidacloprid, these values were found to be 2.31 and 2.18 days, respectively. B-cyfluthrin residues disappeared below the limit of quantification (LOQ) of 0.01 mg kg-1 at single and double application dosages after 5 and 7 days, respectively, however imidacloprid residues took 10 days at both dosages.7.
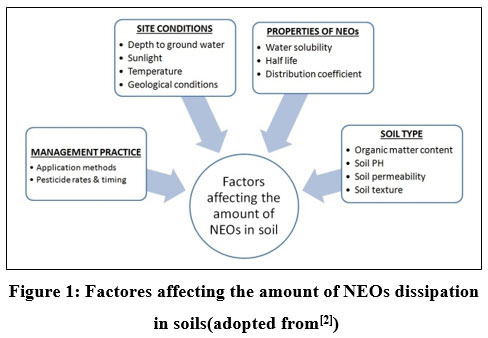 |
Figure 1: Factores affecting the amount of NEOs dissipation in soils(adopted from2 |
Numerous studies have shown that animals including mammals, birds, and pollinators like bees are at risk due to high NEO pollution8-10. For instance, neonicotinoids have been shown to be very hazardous to earthworms, mostly by disrupting their capacity for reproduction, rates of growth, and behavioral characteristics 11.In addition, prolonged contact with NEOs in the food chain can have a negative impact on human health. 12.According to several researches, the physical and chemical factors that affect pesticide dissipation include environmental variables, application techniques, plant species and growth rates, dosage, intervals between treatments, and harvest times. Figure 1 depicts the variable that affects the amount of NEOs dissipation in soils. The determination of the amount of pesticide residue the gas and “GC and HPLC” coupled with “Mass Spectrometry (MS)” has found to be in second and third place, respectively, as compared to conventional extraction techniques. The QuEChERS method has the high analytical scope having highest level of selectivity and sensitivity for analysis. The QuEChERS approach yields extremely promising results for monitoring the samples as compared to other methods13. HPLC is a sensitive and selective method since neonicotinoids frequently have very low concentration levels and their environmental matrices are frequently complex. Preparation of the sample for the analysis is an important task. It is possible to remove additional substances from the sample matrix in addition to the analyte, which can be preconcentrated. Numerous approaches have been recommended for sample pretreatment and/or preconcentration of neonicotinoids in various matrices 14.Varying amounts of pesticide residue are left behind after application in an agro-ecosystem, and the its harmful effects depend on their chemical make-up, degree of metabolism, metabolites toxicity, etc. The present study was carried out to standardize the methodology for measurement of residues, assessment of dissipation kinetics of residues and of IMI and THI after application in sweet lime orchards. This is because there is less information on the persistence and metabolism of IMI and THI in sweet lime orchard soils.
Materials and Methods.
Chemicals and Reagents
Imidacloprid (99% purity) and thiamethoxam (99.8% purity) technical-grade standards were purchased from M/S Chromatopak Analytical Instrumentation Pvt. Ltd., Mumbai. The HPLC-grade solvents acetonitrile, methanol, and QuEChERS extraction salts including sodium sulfate anhydrous, C2H3NaO2, and MgSO4 anhydrous were purchase from local suppliers. To conduct a field experiment, a mixture of thiamethoxam (Syngenta-Allika, 12.6% W/W+) and imidacloprid (Bayer Admire, 35% EC emulsifiable concentrate) was procured from regional agricultural pesticide store in Pulivendula, Kadapadist, A.P.
Sampling and Sample Preparation
Soil samples were taken from the surrounding villages of Pulivendula, Andhra Pradesh in tidy sweet lime orchards. The spraying of IMI and THI was done @ 2 ml/l and 1.5ml/l respectively. About 3 kg of soil samples were taken at intervals of 0 (within one hour), 1, 3, 5, and 10 days after spraying in sealed covers under refrigeration. The samples were stored in refrigerator in a laboratory @ -20oC for subsequent examination. After being air-dried, collected soil samples were passed through a 2 mm sieve. Samples of the properly dried soil were put in falcon tubes. The trial was conducted during hasta season (Sweet lime flowering season) October 2022 with normal agronomic practices as recommended by the regional agricultural office, Tirupati, A.P.
Instrument
Shimadzu’s “Ultra-High Performance Liquid Chromatography (UHPLC)” with the “SPD-M20A PDA,” having “Deuterium” (D2) and “Tungsten (W) lamps,” analyses the sample at a wavelength between 190 nm and 800 nm.
Instrumentation
The method for quantifying IMI and THIs is standardized by using the following UHPLC system parameters. The IMI and THI residues from soils of sweet lime orchards were detected and quantified at various spray intervals, including 0, 1, 3, 5, and 10 days. The XR-ODS II (150 mm x 2.0 mm) was used for chromatographic separation, and the column oven temperature was set at 40°C. For both IMI and THI, the UHPLC conditions were listed in Table 1.
Table 1: UHPLC experimental optimized conditions
| S.No | Parameter | Conditions for IMI | Conditions for THI |
| 1 | Liquid chromatograph | Schimadzu,NexeraX2 SPD – M20A 230V | M20A 230V, Schimadzu, NexeraX2 SPD |
| 2 | Pump | LC-30AD (Low pressure gradient) | LC-30AD (Low pressure gradient) |
| 3 | Column | Discovery ®C18 (15cmX4.6mm,5µm) | Discovery ®C18 (15cmX4.6mm,5µm) |
| 4 | Pressure& Oven Temperature | 261-262kgfm& 35oc | 261-262kgfm& 30oc |
| 5 | Interval &Injection Volume & | For 10 min 10 μl | For 10 min 10 μl |
| 6 | Total flow | 0.4 ml/min | 0.6 ml/min |
| 7 | Mobile phase | Pump A- Water (60%)
Pump B: Methanol (40%) |
Pump A- Water (70%)
Pump B: Methanol (30%) |
| 8 | Wavelength | 272nm | 260nm |
| 9 | Detector | Photodiode array (PDA) | Photodiode array (PDA) |
| 10 | Detector mode | SPD-M30A | SPD-M30A |
| 11 | Lamp | D2 (Deuterium) | D2 (Deuterium) |
| 12 | Total run time | 15 min | 15 min |
| 13 | Retention time | 6.0 min | 4.4 min |
Preparation of HPLC solvents and its standard solutions
The 40 % methanol has prepared by mixing 400 ml of methanol was mixed with 600 ml of HPLC-grade water. A precisely weighed quantity of 100 mg of IMD and THI was separately dissolved in a small amount of methanol solvent and made up of 100 ml of methanol to form solutions that contained 1000 µg/ml of IMD and THI. A calibration curve was plotted after the stock solutions were subsequently diluted to achieve linear values (0.1 to 1.0 µg/ml).
Sample extraction
The IMI and THI were extracted from soils using the QuEChERS technique that was compatible with UHPLC and a PDA detector. A 50 ml centrifuge tube containing fifteen grams of the homogenized soil sample and 30 ml of Acetonitrile (1 percent) concentration was used for analysis. The solution was intermittently centrifuged for three minutes at 15000 rpm for homogenates of the mixture. Then, 1.5 g of sodium acetate and 6 g of Na2SO4 were added to the mixture. To separate the organic layer, the mixture was vortexed for 1 minute and then centrifuged at 2500 RPM for 2 minutes. A 15 ml centrifuge tube was filled with 9 ml of the top organic layer after centrifugation, along with 0.45 g of primary secondary amino acids (PSA) and 1.4 g of magnesium sulphate. The tube was then centrifuge at 2500 RPM for 2 minutes. The filtrate was centrifuged, moved to an LC vial, and then injected straight into the UHPLC auto sampler, yielding 1 millilitre of the filtrate. The, 2 ml of the supernatant layer was then separated, pipetted into a 15-ml centrifuge tube, and passed through a PTFE filter15. The residue was calculated using the equation 1 shown below:
![]()
IMI and THI quantitative analysis
Method development
Linearity and recovery range, LOD (Limit of detection) and LOQ (Limit of quantification), precision, accuracy, and specificity were all validated during the method development process (Using a standard external approach). Calibration of the device is essential for accurate analysis. Linearity is the ability of an analytical procedure to produce test data that are directly proportional to the quantity of an analyte in the sample. It is assessed by using a minimum of five linear concentrations and a regular regression analysis of the diagram of impulses as a function of the concentration of the analyte. The regression line’s slope and correlation coefficient should be satisfactory. The IMI (1000 µg/ml) stock solution was pipetted out, diluted with methanol to create various concentrations, and then injected into UHPLC-PDA. The peak regions were measured and tallied. Standard solutions of IMI and THI (0.1 to 1 µg/ml in mobile phase), which changed the detector response in terms of peak regions, were used to determine it. The amounts of IMI and THI were assumed to create a S/N ratio of 3:1, and the LOQ was calculated using a S/N ratio of 10:1. Based on the chromatogram of the lowest concentration that might cause a 3:1 response after injection of various doses, these numbers were computed. The IMI and THI were administered three times, each at 1 ppm, to assure accuracy.
Method validation
A conical flask containing a 5-g sample of finely mixed soil was then filled with the required amount of standard IMI and THI stock solutions (250 to 500 µg/ml in methanol). These samples were mixed, and air-dried. The IMDs were quantified after the samples were extracted and cleaned.
Dissipation kinetics of Imidacloprid &Thiamethoxam residues in Soil samples
Charting the residue concentration versus the amount of time since spraying (measured in days) was carried out for the estimation of the dissipation kinetics of pesticide residues, and the curves of the best-fitting equations were derived for the best coefficients of determination (R2). It was discovered that the IMI and THI dissipation in soil followed an exponential relationship that corresponded to the general first-order kinetic.
![]()
Where R0 is the initial residue concentration, Rt is the quantity of pesticide residues present at time t, and k denotes the pesticide’s daily dissipation rate, that is constant.
Statistical analysis
The trend of IMI & THI dissipation over time in soils was described using the logarithmic first order kinetics, a derivative of exponential decay. The biological half-lives, or the amount of time needed for the original residue to degrade by one-half was also computed 15 as shown in figure 5.
Results and Discussions
The smallest concentration that provides a satisfactory S/N ratio out of 10 in the chromatographic signal is known as the “limit of quantification (LOQ)”. The standards were prepared to compare S/N ratios, using the sample preparation technique outlined above and then added to the UHPLC apparatus. The optimized chromatographs of IMI and THI were shown in Figure 2 (a) & (b). Peak intensity increased with increasing concentration values from 0.1 ppm to 1 ppm of IMI. The solvent peak (MeOH) was shown in Figure 2 (a). The chromatograms of THI, indicated that the peak intensity increased with increasing concentration values from 0.1 ppm to 1 ppm of THI. The solvent (MeOH) peak was shown in figure 2 (b).
The calibration graphs of IMI and THI in methanol solvent were linear with an index of correlation that was greater than 0.996 within the tested interval of 0.1 to 1 ppm. Figures 3.(a) and (b) shows the linearity graphs of IMI and THI. Spiked soil recovery ranges from 95% to 110%. Comparison between the amounts that were added with those that were found and recovery was achieved and is represented in the bar chart depicts the recovery results, which show that the THI had a higher recovery percentage than the IMI Figure 4. Table 2 displays the calculated LOQ and LOD for IMI and THI, which were 0.451 ppm and 0.150 ppm respectively for IMI and 3.21 and 1.07 ppm respectively.
The earlier research indicated that the rapid degradation of IMI and THI with half-lives of 4.9–5.4 days in vegetable cowpea[4], and 1.25–1.36 days in cardamom [5] provides support for the current study. Lower relative humidity often resulted in less downwind deposition because of evaporative effects. As wind speeds increased, less material was deposited and more mass could not be accounted for in the experiment. Similar to this,16-20 reported that factors such as water solubility, vapor pressure, hydrolysis, microbial activity and environmental factors can affect how pesticides applied to crops degrade. In contrast to the current investigations, higher dissipation tendencies of IMI and THI were noted, and several other researchers found relatively short half-lives for IMI and THI.
Table 2: LOD and LOQ of IMI and THI
| Compound Name | LOD | LOQ |
| IMI | 0.150 ppm | 0.451 ppm |
| THI | 1.07 ppm | 3.21 ppm |
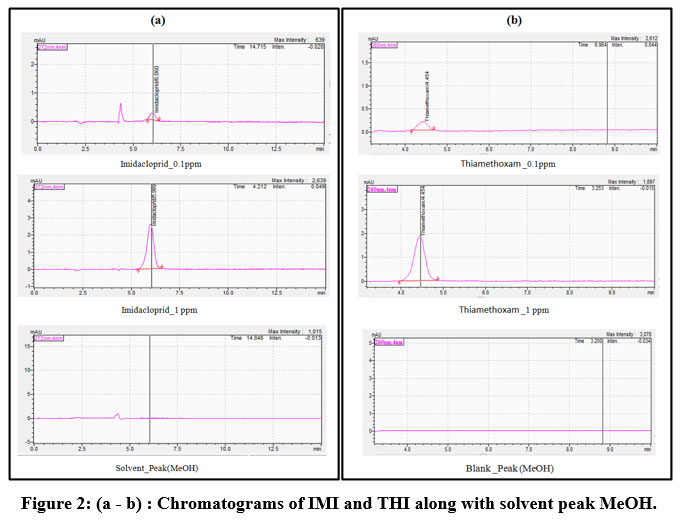 |
Figure 2: (a – b) : Chromatograms of IMI and THI along with solvent peak MeOH. |
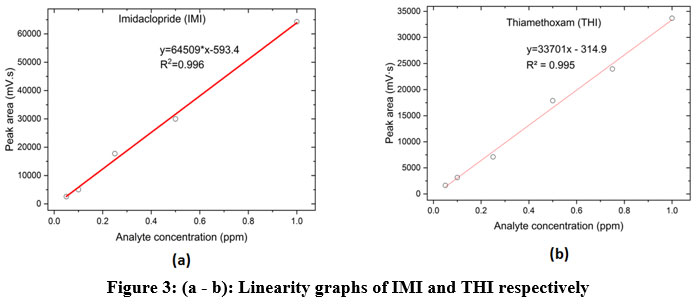 |
Figure 3: (a-b): Linearity graphs of IMI and THI respectively. |
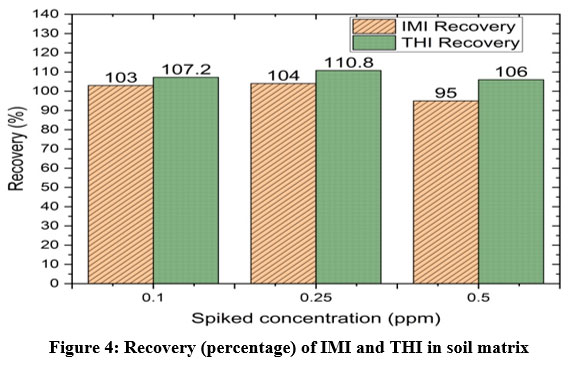 |
Figure 4: Recovery (percentage) of IMI and THI in soil matrix |
Table 3: Dissipation kinetics of IMI and THI
| S.No | Interval | Imidacloprid (IMI) | Thaimethoxam (THI) | ||||||||
| Residues in ppm | |||||||||||
| Days | R1 | R2 | R3 | Mean | Dissipation% | R1 | R2 | R3 | Mean | Dissipation% | |
| 1 | 0 | 1.96 | 1.71 | 2.05 | 1.91 | 00 | 2.00 | 1.94 | 1.88 | 1.95 | 00 |
| 2 | 1 | 0.28 | 0.48 | 0.59 | 0.45 | 76.43 | 0.93 | 0.94 | 0.92 | 0.93 | 52.30 |
| 3 | 3 | 0.30 | 0.33 | 0.30 | 0.31 | 83.76 | 0.31 | 0.31 | 0.36 | 0.32 | 83.58 |
| 4 | 5 | 0.06 | 0.08 | 0.06 | 0.07 | 96.34 | 0.07 | 0.05 | 0.04 | 0.06 | 96.92 |
| 5 | 10 | BDL | BDL | BDL | BDL | BDL | BDL | BDL | BDL | BDL | BDL |
The dissipation percentage of IMI and THI were reported in table 3. Although various environmental mechanisms insecticide degradation, after conducting research, it was shown that volatilization is the primary factor affecting pesticide dissipation in open fields under natural settings. The imidacloprid metabolites such as 5-hydroxy, urea, and guanidine disappeared within short time period, hence there was very little toxic effect was noted 21.
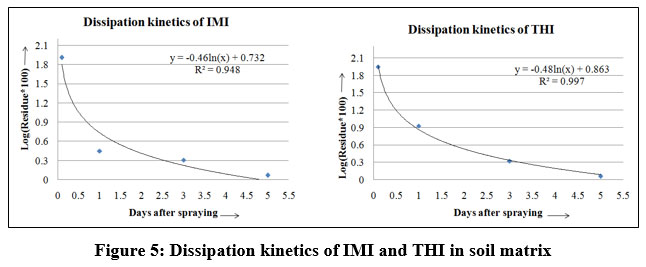 |
Figure 5: Dissipation kinetics of IMI and THI in soil matrix |
The dissipation rates of IMI and THI (total residues) administered at rates of 2 ml/l and 1.5 ml/l, respectively, are shown semi-logarithm graph figure 5. Neonicotinoids residues were discovered in 78% of the samples from 67 topsoil samples from the 3 provinces of Philippi. The soils used for growing rice, bananas, and vegetables, total neonicotinoid doses typically varied around 0.017 – 0.89 μg/kg; whereas, soils used for a citrus grove had higher levels. Imidacloprid was the highest common substance found in soil, with a mean concentration of 0.57μg/kg. It was not detected with thiacloprid22. From figure 5 it was clearly observed that the IMI and THI dissipation in soil followed an exponential relationship, which corresponded to the general first-order kinetic. The current study’s findings showed that at 0 days (1h) after spraying, the first residues of the IMI and THI from soils were 1.91 and 1.95 ppm, respectively. Ten days after the spray, it was dropped below the detectable level as IMI and THI disappeared from soil tests. The standard solution at five different concentrations showed an IMI retention time of 6.0 min. For comparison, a simultaneous blank run with methanol is shown in figure 2 for both the IMI and THI investigations, linear concentrations of 0.05, 0.10, 0.25, 0.50, and 1.00 ppm were employed. The THI retention period was 4.4 minutes. Figure 2 shows the results of running the blank and the methanol comparison tests together.
Conclusion
The IMI and THI residues from sweet lime orchards in Pulivendula, close to Kadapa District, A.P, dissipated in an open field experimental environment. Simple and quick analytical techniques were created and analyzed utilizing a UHPLC to identify the pesticide residues. The biological half-lives of IMI and THI were determined in the field investigation to be 1.35 days and 1.76 days, respectively, in the sweet lime orchards soils. The IMI residues were 1.91, 0.45, 0.31, and 0.07 ppm on days 0, 1, 3, and 5 after spraying, respectively, and were detected below detectable levels on day 10.The coefficient of determination, R2 was 0.948. The THI residues were 1.95, 0.93, 0.32, and 0.06 mg/kg respectively on 0, 1, 3, and 5 days after spraying, and were detected below detectable levels on day 10. The coefficient of determination, R2 was 0.997.
References
- F. J. Byrne and N. C. Toscano, “Characterization of neonicotinoids and their plant metabolites in citrus trees and grapevines, and evaluation of their efficacy against the glassy-winged sharpshooter and the egg parasitoid Gonatocerus ashmeadi,” In Proceedings of the Pierce’s Disease Research Symposium, pp. 287–289, 2005.
- R. Ramadevi and G. V. S. reddy C. Ramachandraiah, “A Review on Contamination of Soil and Water by Neonicotinoid Pesticides and Trends it’s in Soil and Water Samples with Chromatographic Analytical Techniques,” Oriental Journal Of Chemistry, vol. 38, no. 2, pp. 259–267, 2022, doi: 10.13005/ojc/380205.
CrossRef - M. Fossen, “Environmental Fate of Imidacloprid,” Regulation, pp. 1–16, 2006.
CrossRef - B. K. K. Reddy, A. Paul, and T. George, “Dissipation Kinetics and Risk Assessment of Thiamethoxam 25 % WG Residues in Vegetable Cowpea,” Indian Journal of Entomology, vol. 84, no. 2, pp. 449–452, 2022, doi: 10.55446/ije.2021.265.
CrossRef - N. Pratheeshkumar N. Chandran, M. Beevi, S. Naseema Mathew, Thomas Biju George, Thomas Paul, Ambily Xavier, George Ravi, K. Prathibha Kumar, S. and Visal Rajith, R., “Dissipation kinetics and effect of processing on imidacloprid and its metabolites in cardamom (Elettaria cardamomum Maton),” Environmental Monitoring and Assessment, vol. 188, no. 1, pp. 1–14, 2016, doi: 10.1007/s10661-015-5058-5.
CrossRef - J. Zhou Zhou, Jie Dong, Chao An, Wenjing Zhao, Qiyang Zhang, Yaohai Li, Zhixia Jiao, and Bining “Dissipation of imidacloprid and its metabolites in Chinese prickly ash (Zanthoxylum) and their dietary risk assessment,” Ecotoxicology and Environmental Safety, vol. 225, p. 112719, 2021, doi: 10.1016/j.ecoenv.2021.112719.
CrossRef - N. S. Moghaddam, M. P. Zakaria, D. Omar, and K. Sijam, “Extraction Efficiency and HPLC Determination of Imidacloprid in Soil,” Soil and Sediment Contamination, vol. 21, no. 8, pp. 985–995, 2012, doi: 10.1080/15320383.2012.712071.
CrossRef - C. A. Hallmann, R. P. B. Foppen, C. A. M. Van Turnhout, H. De Kroon, and E. Jongejans, “Declines in insectivorous birds are associated with high neonicotinoid concentrations,” Nature, vol. 511, no. 7509, pp. 341–343, 2014, doi: 10.1038/nature13531.
CrossRef - E. A. D. Mitchell, B. Mulhauser, M. Mulot, and A. Aebi, “A worldwide survey of neonicotinoids in honey,” vol. 111, no. October, pp. 109–111, 2017.
CrossRef - Z. Zeng, G.; Chen, and M.; Zeng, “Risks of Neonicotinoid Pesticides,” Science, vol. 340, no. 6139, pp. 1403–1403, 2013, doi: 10.1126/science.340.6139.1403-a.
CrossRef - K. Wang Wang, Kai Pang, Sen Mu, Xiyan Qi, Suzhen Li, Dongzhi Cui, Feng Wang, and Chengju “Chemosphere Biological response of earthworm , Eisenia fetida , to five neonicotinoid insecticides,” CHEMOSPHERE, vol. 132, pp. 120–126, 2015, doi: 10.1016/j.chemosphere.2015.03.002.
CrossRef - A. M. Cimino, A. L. Boyles, K. A. Thayer, and M. J. Perry, “Review Effects of Neonicotinoid Pesticide Exposure on Human Health : A Systematic Review,” Environmental Health Perspectives, vol. 125, no. 2, pp. 155–162, 2017.
CrossRef - P. A. Souza Tette, L. R. Guidi, M. B. De Abreu Glória, and C. Fernandes, “Pesticides in honey: A review on chromatographic analytical methods,” Talanta, vol. 149, pp. 124–141, 2016, doi: 10.1016/j.talanta.2015.11.045.
CrossRef - M. Farouk, L. A. E. A. Hussein, and N. F. El Azab, “Simultaneous determination of three neonicotinoid insecticide residues and their metabolite in cucumbers and soil by QuEChERS clean up and liquid chromatography with diode-array detection,” Analytical Methods, vol. 8, no. 23, pp. 4563–4575, 2016, doi: 10.1039/c6ay01161f.
CrossRef - T. Murali Krishna, K. Devaki, K. Kiran Kumar, and L. Prasanthi, “Dissipation kinetics and the pre-harvest residue of chlorantraniliprole in pigeon pea Cajanus cajan L. succulent pods Using Ultra-High-Performance Liquid Chromatography with Photodiode array detector (UHPLC-PDA),” Open Journal of Analytical and Bioanalytical Chemistry, vol. 6, no. 1, pp. 013–017, 2022, doi: 10.17352/ojabc.000025.
CrossRef - D. K. Tewary, V. Kumar, S. D. Ravindranath, and A. Shanker, “Dissipation behavior of bifenthrin residues in tea and its brew,” Food Control, vol. 16, no. 3, pp. 231–237, 2005, doi: 10.1016/j.foodcont.2004.02.004.
CrossRef - M. Fujita, T. Yajima, K. Iijima, and K. Sato, “Comparison of the variability in the levels of pesticide residue observed in Japanese cabbage and grape units,” Journal of Agricultural and Food Chemistry, vol. 60, no. 6, pp. 1516–1521, 2012, doi: 10.1021/jf2040059.
CrossRef - N. Sharma, K. Mandal, R. Kumar, B. Kumar, and B. Singh, “Persistence of chlorantraniliprole granule formulation in sugarcane field soil,” Environmental Monitoring and Assessment, vol. 186, no. 4, pp. 2289–2295, 2014, doi: 10.1007/s10661-013-3537-0.
CrossRef - M. Paramasivam, “Dissipation kinetics, dietary and ecological risk assessment of chlorantraniliprole residue in/on tomato and s[1] M. Paramasivam, Journal of Food Science and Technology 2021, 58, DOI: 10.1007/s13197-020-04573-5.oil using GC–MS,” Journal of Food Science and Technology, vol. 58, no. 2, pp. 604–611, 2021, doi: 10.1007/s13197-020-04573-5.
CrossRef - C. Sun, K. Bei, Y. Xu, and Z. Pan, “Effect of Biochar on the Degradation Dynamics of Chlorantraniliprole and Acetochlor in Brassica chinensis L. And Soil under Field Conditions,” ACS Omega, vol. 6, no. 1, pp. 217–226, 2021, doi: 10.1021/acsomega.0c04268.
CrossRef - S. Celika and T. Asanb, “Degradation of some pesticides in the field and effect of processing,” Analyst, vol. 120, no. June, pp. 1739–1743, 1995, doi: 10.1039/an9952001739.
CrossRef - J.-M. Bonmatin et al., “Residues of neonicotinoids in soil, water and people’s hair: A case study from three agricultural regions of the Philippines,” Science of The Total Environment, vol. 757, p. 143822, 2021, doi: https://doi.org/10.1016/j.scitotenv.2020.143822.
CrossRef

