Introduction
During the last couple of decades usages of chemicals are protect crops against infectious diseases, however, they have a leading adverse influence into the soil environment, human health and ecological disturbance. Due to the negative impact and increasing cost of chemical fertilizers, utilization of beneficial soil microorganisms for sustainable agriculture is the necessity of the world. Plant Growth Promoting Rhizobacteria (PGPR) is a heterogenous group of bacteria found at a root surface of the rhizospheric soil and they can be associated with roots that are able to improve the quality of plant growth by directly or indirectly. The interaction between plant and microbes can be beneficial and utilized for plant growth improvement and enhance the crop productivity.9,39 The major application of PGPR to enhance the plant growth includes agriculture, horticulture, forestry and environment restoration without usage of chemical fertilizers. For the protection of seeds, bio-priming is a revolutionary approach for plant growth promotion and crop protection. Seed bio-priming is the method to promote seed germination, enhance stress tolerance capacity, plant growth regulation, and acting as a bio-control agent that provoke plant defense mechanism.33 In seed bio-priming process it can prevent the plumule and radicle development from the adverse environmental effect and plant pathogens before seed is sown.6 The present study was focus on the screening of free-living soil bacterial (Bacillus sp. OQ654027) strain facilitate the seed bio-priming technique that can improve and enhanceplant-growth-promotion activity of groundnut and chickpeafor sustainable agricultural practices.
Materials and Methods
Collection of Sample and Processing
Soil collected for the pot experiments belongs to the “black soil” from the agricultural field of Khijadiya, Dist. Rajkot, Gujarat. Targeted soil has neutral in nature.14 Collected soil is categorized as clay within 40-50cm of the surface and it is neutral in nature. The pH value, and electrical conductivity (EC) of bulk soil samples were 7.65 and0.54ppm respectively. This soil contained 0.51ppmtotal nitrogen organic carbon (OC)%, 15 ppm phosphorus (P) and 116 ppm soil exchangeable potassium (K), microelements such as 5.47 ppm Zn, 1.00 ppm Fe, 5.74 ppm Mn and 5.04 ppm Cu. Data shows the analysis of soil quality is high to low in the presence of micronutrients and in macro elements present in the soil is medium to low level.
Isolation and Identification of Bacterial Strain
Isolation of Rhizobacteria from Processed Selected Soil Sample
The rhizospheric soli was collected from the crop fields of Khijadiya, Saurashtra region, Gujarat. The soil samples were placed in Ziploc bags and store at 40°C for futuristic studies. For the isolation of bacterial strain collected soil sample were serially diluted followed by 10-1 to 10-6 and inoculated on nutrient agar plates and incubated at 37°Cfor 24 – 48 hours. Single colony was picked up and streaked on sterile nutrient agar plate to get pure culture. Pure isolated, purified colonies were observed for morphological and microscopic characterization and maintained for further studie.5
Characterization of Selected Bacterial Stain (KC9)
In the present investigation, the isolated bacterial strain was studied for microscopic, morphological, and biochemical characteristics based on Bergey’s Manual of Systematic Bacteriology. Morphological characterization viz. colony morphology (size, shape, margin, elevation and consistency, opacity and colour) and gram’s nature and arrangement of recovered isolates examined by Gram’s staining. For the genus identification involving biochemical analysis are IMViC test, Sugar fermentation (Dextrose and Mannitol) followed by the Starch utili.2
Microscopic Identification
Gram’ s Staining overview
A single colony was taken from the young culture plate and uniformly placed on a glass slide to prepare a smear employing crystal violate for 1 minute followed by gram’s iodine then washed with the decolorizing agent (95% ethanol) then added basic dye (safranin)for another 1 minute to wash with sterile water allowed to air dry and then observed under microscope at 100X.2
Biochemical Characterization
Indole Test
Tryptophan broth was employed to check the presence of tryptophanase enzyme into bacterial strain. Bacterial culture was inoculated on to the broth medium and incubated at 37°C for 48 hours reaction has been completed by adding Kovac’s reagent to the tubes examine the positive indole test is indicated by the formation of cherry red ring in the reagent layer on top of the medium.2
Methyl-Red Test
Bacterial culture was inoculated to the nutrient broth medium containing peptone, 5.0 g; glucose, 5.0 g; NaCl, 5.0 g; distilled water, 1000 ml; pH, 7.0 and incubated at 37°C for 24-48hours. Formation of bright red colour was indicator of positive result after adding 3-4 drops of methyl red reagent was added to each tube.23
Voges-Proskauer Test
VP broth was inoculated with bacterial isolates and incubated at 37°C for 48 hrs followed by the addition of 10-12 drops of 5% α-naphthol and 6-8 drops of aqueous KOH solution then shaken well for the formation of cherry red ring which indicate positive result.23
Utilization of Citrate Test
Simmon’s citrate broth was employed by examine the ability to use citrate from organisms. Inoculated the pure bacterial isolate on to the slant then incubated at 37°C for 48 hours. Results were recorded after observing the growth and alkaline reaction. Bacterial growth would be visualized on to the slant surface and the medium colour has been will be changed from green to intense prussian blue. Only those bacteria can utilize citrate as the sole carbon and energy source would be able to cultivate on the simmons citrate medium.38
Starch Hydrolysis
Bacterial strain wasgrown onto the surface of the starch agar after24 hrs. of incubation, the surface of the plate was submerged with iodine to detect the presence or absence of starch in the vicinity around the bacterial growth by revealed as a clearing zone surrounding the isolated culture.12
Catalase Activity
This test determines the ability to degrade hydrogen peroxide by secretion of catalase enzyme. One drop of 3% hydrogen peroxide is added to the sterile glass slide followed by bacterial colony and assorted well. The production of air bubbles indicates the presence of catalase observed the result carefully.19
Motility Test
Used to detect the ability of bacterial movement away from the inoculation line. The bacterial colony was inoculated into the soft agar medium through a needle and incubated at 37°C for 24 to 48 hours, to observe the migration.19
Carbohydrate Fermentation
Phenol red broth containing specific carbohydrate source for media completion. Mature bacterial culture was inoculated into the tubes and incubated at 37°C for 48 hours. After the completion of incubation period acid production that indicates lowering the pH of the test medium, that detected by the color change of the pH indicator by change the colour red to yellow and to determine the gas production durum’s tubes were utilized.16
Molecular Identification of KC9:
16S RNA Sequencing
For molecular identification, a mature culture of KC9 has been outsourced at Gene Explore Diagnostics & Research Centre Pvt. Ltd. Ahmadabad, Gujarat for 16S RNA sequencing. As per the std protocol of Sanger’s method, DNA sequencing reaction of PCR amplicon was carried out with 357F & 1391R primers using BDT v3.1 Cycle Sequencing Kit on ABI 3500xl Genetic Analyzer. The partial 16s rRNA sequence was used to carry out BLAST with the database of NCBI GenBank database. Based on the maximum identity score first ten sequences were selected and aligned using multiple sequence the alignment software programs.9
In-vitro Screening to Growth Promotion Analysis of PGPR Trait
Production of Ammonia
The bacterial isolates were tested for the qualitative production of ammonia 24 hr. old bacterial culture inoculated to peptone water medium and incubated at 37°C for 48 hours followed by the addition of 1.0ml of Nesseler’s reagent to each tube to detect the ammonia production by colour development. The development of yellowish-brown color in the test tube indicates the production of ammonia.15
HCN Production
HCN production is the major factor to suppress pathogens from plants. The bacterial culture was inoculated on a nutrient medium supplemented with 4.4 g/l glycine. Sterile filter paper saturated with picric acid solution (2.5 gm of picric acid, 12.5 gm of Na2CO3, 1000 ml d/w) was placed on the upper lid of petri-plateand incubated at 37°C for 48 hours. Observing the colour change of filter paper from yellow to light brown, brown-reddish, and brown was recorded as weak, moderate, and strong respectively.31
Indole Acetic Acid Production
The bacterial culture has been inoculated in the tryptophan broth medium and incubated 37°C for 48 hours after the incubation, centrifuged the sample at 10000rpm for 5 mins and collect the supernatant into the fresh test tube then added 2.0 ml of 2ml Salkowski followed by the 30 mins of incubation at room temperature. Colour change (pink) indicated a high amount of IAA production, it recorded by the absorbance at 530nm.22
Siderophore Production
A qualitative assay of siderophore production was conducted in Chrome Azurole’s (CAS) agar medium. CAS medium were prepared and spot inoculate with bacterial isolates and incubated at 37°Cfor 3-5 days. Development of yellow – orange halo around the colony was considered siderophore production.31
Inoculum Preparation
The PGPR (Bacillus sp.) strain was inoculated in 100 Erlenmeyer flask containing 50 ml nutrient broth and incubated at 37°C for 2 days. During inoculation, the viable cell suspension count was 33 x 108 CFU/ml.11
Seed Treatment
PGPR (Bacillus sp.) strain was employed in groundnut and chickpea seed treatments. Seeds were purchased from the local market of Rajkot region. All the seeds were surface sterilized with 0.02% of sodium hypochloride solution for about 2-3 mins then rinsed thoroughly with double distilled water. For seeds inoculation, dipped all the seeds into the bacterial (108 cfu ml–) suspension for 2 mins and considered as treated seeds while only seeds soaked with double distilled water as control non-treated.27
Bioassay of Seed Germination
A seed germination assay was carried out by employing the paper towel method.28 5-5 seeds were selected for each treatment designed for the distribution of the experiment employing a completely randomized design with three replicas, as the petri dish is an experimental component and incubated in moist chamber at 28°C for 7 days. Observed and counted the germinated seed numbers such as groundnut and chickpea. After incubation, germination index and relative seed germination of seedlings root and shoot length were assessed by employing formula.13
![]()

Pot Culture Analysis
For the evaluation of groundnut and chickpea seedlings growth promotion with Bacillus sp. was tested with the sterile and non-sterile soils in the Microbiological laboratory at Atmiya University. For the testing paper glasses were utilized with the capacity of 50gm of soil. All the treatments arrange in 60 pots i.e., 3 replicas of 12 pots. Treatment was arranged based on a completely randomized design. After 15 days of root length, shoot length, number of leaves and chlorophyll content has been evaluated.27
Effect of Seedling Germination With and Without Treatment of Bacillus sp. (KC9) on Groundnut and Chickpea:
Collection of treated Groundnut & Chickpea Seedlings
The chickpea groundnut plant leaves was collected from the pots for further analysis. Plant seedlings were grown for 15 days. Three plants were analyzed for growth parameters. Different morphological parameters were studied like root length, shoot length and fresh and dry weight of leaves.
Total Chlorophyll Content
Total chlorophyll content, content of chlorophyll a and chlorophyll b were determined by using Arnon (1949).One gram of groundnut and chickpea leaves were taken ,then finely cut and gently chopped in clean mortar pestle with chilled 80% acetone (v/v).For the centrifugation, adjust the volume 25 ml with 80% acetone, then centrifuged at 3000 rpm for 15 minutes. The clear suspension was transfer in a fresh tube and the OD was recorded at 645 nm and 663 nm. The blank was taken as 80% acetone (v/v). The content of chlorophyll a and chlorophyll b was calculated by using the following formula.29
Where,
Chlorophyll “a” (µg/ml) = (12.7 ×OD at 663 nm) – (2.69 ×OD at 645 nm)
Chlorophyll “b” (µg/ml) = (22.9 ×OD at 645 nm) – (2.69 ×OD at 663 nm)
Total Chlorophyll (µg/ml) = (20.2×OD at 645 nm) + (8.02 ×OD at 663 nm)
Relative water Content (RWC)
The percentage of relative water content (RWC) was measured using the protocol proposed by.29 The plant leaves were collected randomly to measure the fresh weight, and then the leaves were immersed in a tube with distilled water and put in a fridge to incubate for 24 h. The leaves were blotted to dry and measured for weight when fully turgid. The leaves were dried in an oven for 24 h at 72°C. Lastly, the dry weight was recorded, and the percentage of relative water content was measured using the following formula:
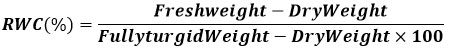
Total Free Amino Acids
Plant tissue extract was made up to 1 ml with distilled water and ninhydrin reagent was added. The test tubes were put in the boiling water-bath for 20 minutes. The test tubes were cooled and 5.0ml of diluent was added in the each tube and the absorbance was recorded at 570 nm.29
Total Phenolic Content
The total phenolic content were analyzed by using Folin- Ciocalteu (FC) reagent using the method of Ainworth and Gillespie. The seed sample 0.5 g was homogenized with 500 µl of 95% chilled methanol, after the sample was incubated at room temperature for 48 hours. The sample was centrifuged for 5 minute, after the centrifugation the supernatant was collected in fresh tube and the TPC was estimated by adding 100 µl FC reagent. The mixture was vortex and adding the 800 µl Na2CO.The sample was incubated for 1 hour and after taking the absorbance at 760nm using a spectrophotometer. The Gallic acid standard curve was prepared and the result were presented as mg GAE/100g.26
Total Flavonoid Content
The total flavonoid content of seeds was determine by the aluminium chloride method. First prepare the sample. Take the 5 ml of sample and then added to 2% Alcl3 5 ml in each tube, incubated the tubes for 20 minutes. After the incubation, taken the absorbance at 415 nm using the spectrophotometer. Quercetin standard curve was used and the results are expressed in mg QE/100g.7
Statistical Analysis
The experiments were performed in triplicates and the results have estimated using mean ± standard deviation and One-way analysis of variance to determine the effect of bacterial isolate on groundnut, chickpea and bean seeds and seedling germination. To evaluate the significant difference between two means paired T- test has been applied at p ≤ 0.05using statistical package WAS 1.0, ICAR, Goa.
Results& Discussion
Isolation and Microscopic Examination of Bacillus sp. (KC9)
Total 11 colonies were observed from black soil from Khijadiya village, at Saurashtra region, Gujarat. Among 11 colonies two are showing PGPR activity. The isolated colony was identified as Bacillus sp. by the morphological and microscopic examination. The isolate abbreviation is KC9 as per the location of Khijadiya chickpea crop filed. By the morphological identification KC9 colony elevation showed to be flat in nutrient agar medium. It is having small size and round in edges by showing smooth consistency and opacity was opaque in nature contains white in colour with respective of table-1. By employing Gram’s reaction and in microscopic identification Bacillus isolates was gram positive (+ve), purple in colour due to the thick peptidogly can layer and rods shape has observed at 100X of oil immersion in fig.1. Similarly, 18 reported various genera like Bacillus, Pseudomonas, Serratia and Enterobacter, etc. by using serial dilution followed by pure plating method for bacterial colonies isolation and identification through gram’s staining and biochemical characterization.
 |
Figure 1: Isolation and Microscopic Identification of Bacterial Culture (KC9). |
Biochemical Characterization of Bacillus sp. (KC9)
By the performance of biochemical characterizations result revealed in fig. 2 and table-1, represented thatKC9 isolate showed positives results for indole, methyl-red, catalase, motility, dextrose fermentation and starch utilization give positive tests, while KC9 can’t able to utilize citrate, tryptophan (VP test) and mannitol as substrate for their growth. 37 reported as Bacillus sp. shows positive results of all the biochemical analysis such as catalase, urease, oxidase, citrate utilization, vogues-proskauer, nitrate reduction test, triple sugar iron agar test, motility and amylase production.
Table 1: Morphological & Biochemical Characteristics of Bacterial Isolate(KC9)
| Morphological Characteristics | Bacterial Isolate (KC9) |
| Size | Small |
| Shape | Round |
| Margin | Entire |
| Elevation | Flat |
| Consistency | Smooth |
| Opacity | Opaque |
| Colour | White |
| Gram’s nature | +ve |
| Organism shape | Rod |
| Name of Microorganism | Bacillus sp. |
| Biochemical Characteristics | |
| IMViC Test | |
| Indole | + |
| MR | + |
| VP | – |
| Citrate | – |
| Starch Hydrolysis | + |
| Catalase Test | + |
| Motility | + |
| Sugar Utilization | |
| Dextrose | + |
| Mannitol | – |
 |
Figure 2: Biochemical Characterization of Bacillus sp. (OQ654027). |
Molecular Identification of Potent PGPR (KC9)
Bacterial Sample KC9 is closely related to Bacillus Species based on nucleotide homology analysis. Results is confirmed by 16s Microbial Screening Genetic Typing using software’s considering E value which shows the 100% match with various Bacillus sp. The evolutionary history was inferred using the Neighbor-Joining method. The optimal tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. There was a total of 847 positions in the final dataset. Evolutionary analyses were conducted in MEGA7. 16S rRNA gene sequences by the comparison of available sequences in the database of gene bank with the help of BLAST homology. KC9 was identified as Bacillus sp. and deposited to the Gene Bank database with accession number of OQ654027. Similarly, as per the study of 33 neighbor-joining method has been utilized for the reconstructing phylogenetic trees and 21 used the MEGA7 bioinformatics tool for molecular evolutionary genetics analysis.
 |
Figure 3: Phylogenetic Tree of Bacillus sp. employing Neighbor-Joining method with Accession number ofOQ654027 |
In-vitro Screening to Growth Promotion Analysis of PGPR Trait
KC9 having the ability to produce siderophores and HCN was represented in the fig. 4 and table-2 respectively. KC9 showed an orange halo after 10 days of incubation on a CAS medium which considered as siderophore-producing bacteria. For HCN production, KC9 (Bacillus sp.) showed positive result by observing the colour change of filter paper from yellow to brown-reddish colour represent the strong producers of HCN that can suppress the plant pathogens. 14, found the similar results in various isolates of AF4II5, AF7II3, and PC3 exhibited the maximum IAA production. Serratia plymuthica and Pseudomonas putida both have the ability to produce HCN and siderophore activity to enhance seed and seedling germination. The ability to convert insoluble nitrogen to solubilize form which can take plant for their growth promotion (Sharma et al., 2021). In the current investigation in fig. 4 represents, KC9 has ability to convert organic nitrogen from peptone into ammonia. A similar drift was represented in rhizospheric bacterial strains isolated from the root soil of cotton and chick pea.17 The phosphate solubilization of KC9 contains the ability to form clear zone around the bacteria on Pikovskaya agar medium containing tricalcium phosphate (Figure 4). KC9 has the ability to form 2.2 mm diameter of the clear zone around on medium, and is the dissolution index is 0.78 mm. Production of IAA through the KC9 bacterial colony that indicates the ability to convert insoluble phosphate to soluble form and fixed atmospheric nitrogen in soil. Indole Acidic Acid production capacity is determined in KC9 by observing intensity of pink colour change that indicate bacterial colony to utilize tryptophan with the concentration of IAA was 1.15 mgl-1. Similar study has been reported by 19 in their investigation a wide variety of morphologically distinct bacterial isolates showed the phosphate solubilization and production of IAA enzyme that able to promote the plant growth characters of maize.
Table 2: Growth promotion analysis of Bacterial Isolate (KC9)
| Growth Promotion Characteristics | Bacterial Isolate (KC9) |
| Ammonia production | + |
| IAA Production | + |
| HCN Production | + |
| Siderophore Production | + |
| Phosphate Solubilization | + |
 |
Figure 4: Growth Promotion Study of PGPR Trait Bacillus sp. (KC9) |
Study the Effect of Seed and Seedling Germination of Groundnut and Chickpea:
For the seed germination, all plant required favorable environmental conditions such as optimum temperature and moisture to breakdown the exogenic dormancy. Hormones, can affect the rate of seed germination and maturity.3 Groundnut and chickpea contain the high protein rich source as food materials. From an economic aspect, in India seed lose is higher in level. Furthermore, need to enhance the rate of seed germination to maintain the crop yield.24 In the present study, the ability of selected isolate (Bacillus sp.– KC9) to promote groundnut and chickpea seed germination was investigated through biopriming method. 5-5 seeds of groundnut and chickpea were soaked to the bacterial suspension of KC9 and placed on the blotting paper containing petri-plate to avoid the dehydration for 7 days in fig. 5 respectively. The adding of the bacterial suspension to seeds that can support the colonization process and increase the bacterial cell number that provide as adhesiveness to seeds. Table 3 represented the seeds germination index and relative seed germination in the presence of potential bacterial suspension and fig 5represented the seed germination within 4 days with the biopriming treated seeds.
Table 3: Relative Seed Germination & Germination Index of Various Seeds
| S. No. | Name of Seeds | Total Number of Seeds | Number of Seeds Germinated | Seed Germination Index | Relative Seed Germination |
| 1. | Groundnut | 05 | 05 | 100% | 100% |
| 2. | Chickpea | 05 | 05 | 100% | 100% |
 |
Figure 5. Effect of PGPR Bacillus sp. strain on Seed Germination of Dicot Plants |
Effect of PGPR strain Inoculant on Chickpea and Groundnut Seedlings
Rhizobacteria having the significant assets to enhance the plant growth promotion and improve plant health.20 Several investigators4,25,40 observed and reveled the ability of microorganisms, to increase rate of seed germination, expand seedling appearance, develop to stress tolerance factors, plants growth yield and promote defense mechanism against various pathogens. Similar reports had been displayed in other crops such as potato, radish plants, pearl millet, wheat, chickpea etc.8,28,36 Similar study has been reported by 10 evaluated the incorporation effect of PGPR Azospirillum brasilense on growth yield of spring wheat. Another similar finding by 9 who studied Escherichia coli and Pseudomonas fluorescens shows the PGPR activity on the growth of Cicer arietinum L. (chickpea).
 |
Figure 6: Growth Promotion Activity: With and Without Treatment of PGPR Strain (Bacillus sp.) |
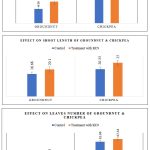 |
Figure 7: Graphical Representation; In-vitro Pot Analysis of PGPR strain on Chickpea and Groundnut Seedlings |
In the present investigation prepared the bio-priming seeds of chickpea and groundnut treated with the 2.0ml each of PGPR strain (Bacillus sp.) while another 5-5 seeds were separately inoculated in the soil only soaking with double distilled water considered as control and sown both treated and untreated seeds into the soil containing plastic glasses in the triplicate manner in seedlings germination for 15 days at under observation. After completion of incubation period recorded root length, shoot length and leaves numbers of germinated seedlings. Result revealed at fig. 6 and fig. 7 that the average of PGPR inoculant treated root length of chickpea is 14.0±2.0cm and groundnut (9.16±1.25cm) while shoot length of treated seeds of chickpea 25.0±2.0cm and groundnut (20.1±1.0cm) and number of leaves in chickpea is 67.33±3.05cm and groundnut (28.3±2.64cm) whereas growth obtained in control seeds; root length of chickpea (10.6±1.25cm) and (6.06±0.90cm) groundnut; shoot length are 20.33±1.0cm and 16.66±1.52cm chickpea and groundnut, similarly in leaves number 61.66±3.51cm and 20.33±2.08cm of chickpea and groundnut respectively. From the statistical approach of paired T-test significant differences at 5% level have been observed in all the parts of the groundnut and chickpea with and without treatment of KC9 (Bacillus sp.) on both the seedlings. By employing the pot experiment with statistical approach, Bacillus sp. (OQ654027) inoculant is considered as plant-growth-promoter that significantly enhance the growth of chickpea and groundnut by early seedling development.
Effect of Seedling Germination with and without Treatment of Bacillus sp. (KC9) on Groundnut and Chickpea.
 |
Figure 8: Graphical Representation of Total chlorophyll content, Relative Water Content (RWC), Total free amino acids, Total Phenolic acid content, Total flavonoid content present in Groundnut & Chickpea Seedlings |
In the present study prepared bio-priming seeds of chickpea and groundnut treated with the 2.0ml each of PGPR strain (Bacillus sp.- KC9) while another 5-5 seeds were separately inoculated in the soil only soaking with double distilled water considered as control and shown both treated and untreated seeds into the soil containing plastic glasses in the triplicate manner in seedlings germination for 15 days under observation. After completion of incubation period, under uninoculated condition showed a significant reduction in chlorophyll a content recorded in the groundnut is 3.585 µg/g, chickpea is 2.975 µg/g, while inoculated condition (treated with KC9) 4.36 µg/g and 3.878 µg/g respectively. The chlorophyll b content increase in the inoculated condition showed in groundnut is 6.16µg/g and chickpea is 4.31 µg/g respectively, when inoculated condition (treated with KC9) recorded 7.71 µg/g in groundnut and 6.76 µg/g in chickpea. The total chlorophyll content is present in groundnut and chickpea under uninoculated is 9.53 µg/g and 7.12 µg/g respectively, under inoculated (treated with KC9) condition 11.81 µg/g and 10.40 µg/g obtained in groundnut and chickpea. Under the uninoculated condition, the relative water content (RWC) of groundnut and chickpea is 68% & 47% estimated, while in treated condition showed in groundnut is 86% & chickpea is 83%. Among all selected legumes, non-germinated chickpea showed lower total free amino acids is 37.5±0.02µg/ml whereas 92.14±0.03µg/ml was observed in groundnut. The graphical representation showed maximum change in groundnut is 304.28±0.03 µg/ml, while the chickpea showed the least difference is 173.2±00.1 µg/ml respectively. The total phenolic content of groundnut measured as 29.62±0.03 mg GAE/100g and chickpea is 20.88±0.01mg GAE/100g, when inoculated condition groundnut measured as 76.28±0.03mg GAE/100g and chickpea is 52.83±0.08 mg GAE/100g respectively. The total flavonoid content was estimated 1.452±0.02 QE/100g in groundnut and chickpea is 0.032±0.01QE/100g under uninoculated condition, whereas inoculated with KC9, 626±0.05QE/100g is recorded in groundnut seedlings and chickpea is 0.085±0.01QE/100g respectively. Similar report of the Adhikari et al., (2019) of his investigation on peanut shells showed remarkable changes in flavonoid (142.6–568.0 mg quercetin equivalents/g), and amino acid (5.76–34.56 mg/g) contents activity.
Conclusion
From the current study it was established that plant growth promoting rhizobacteria (viz. Bacillus sp. KC9) isolated from the rhizospheric soil of chickpea region potentially increase the root, shoot length and leaves numbers of chickpea and groundnut respectively. Therefore, it is considered that the utilization of PGPR strain as active biofertilizer that can help to promote groundnut and chickpea cultivation and one step ahead to reach sustainable crop development.
Acknowledgments
The authors would like to acknowledge the financial assistance from Shodh fellowship, provided by Gujarat State Biotechnology Mission, Gujarat, India and for Research work fulfillment Department of Microbiology, Atmiya University, Rajkot, Gujarat.
Conflict of Interest
All authors are declared that no any conflict of interest in any financial, personal and research work.
References
- Adhikari B., Dhungana S. K., Muhammad Waqas Ali., Adhikari A., Il-Doo Kim., Dong-Hyun S. Antioxidant activities, polyphenol, flavonoid, and amino acid contents in peanut shell. Journal of the Saudi Society of Agricultural Sciences, 2019; 18(4): 437-442.
CrossRef - Aneja K. R., Experiments in Microbiology, Plant Pathology, and Biotechnology, 4th Ed. New Age International, New Delhi, 2005.
- Begum N., Hasanuzzaman M., Li Y., Akhtar K., Zhang C., Zhao T. Seed Germination Behavior, Growth, Physiology and Antioxidant Metabolism of Four Contrasting Cultivars under Combined Drought and Salinity in Soybean.Antioxidants,2022;11(3):498.
CrossRef - Bent E., Tuzun S., Chanway C. P., Enebak S. Alterations in Plant Growth and in Root Hormone Levels of Lodgepole Pines Inoculated with Rhizobacteria. Canadian Journal of Microbiology, 2001; 47(9): 793-800.
CrossRef - Bhatt S., Pandhi N., Raghav R. Improved Salt Tolerance and Growth Parameters of Groundnut (Arachis Hypogaea) Employing Halotolerant Bacillus Cereus SVSCD1 Isolated from Saurashtra Region, Gujarat. Ecol. Environ. Cos, 2020; 26: S199-S212.
- Bisen K., Keswani C., Mishra, S., Saxena A., Rakshit A., Singh H.B. Unrealized Potential of Seed Biopriming for Versatile Agriculture. Springer, 2015:193–206.
CrossRef - Brar D. S., Nayik G. A., AggarwalA. K., Kaur, S., Nanda V., Saxena S.,Gautam S.,Ramniwas S.,Tolcha T. D. Chemical and Functional Characteristics to Detect Sugar Syrup Adulteration in Honey from Different Botanical Origins. International Journal of Food Properties, 2023;26(1):1390-1413.
CrossRef - Burr T. J., Schroth M. N., Suslow T. Increased Potato Yields by Treatment of Seed Pieces with Specific Strains of Pseudomonas fluorescens and Putida. Phytopathology, 1978; 68(9): 1377-1383.
CrossRef - Dasgupta, D., Ghati A., Sarkar A., Sengupta C.,Paul G. Application of Plant Growth Promoting Rhizobacteria (PGPR) Isolated from the Rhizosphere of Sesbania Bispinosa on the Growth of Chickpea (Cicer arietinum L.). Int J Curr Microbiol App Sci, 2015; 4(5):1033-1042.
- Dobbelaere S., Croonenborghs A., Thys A., Ptacek D., Vanderleyden J., Dutto P., Labandera-Gonzalez C., Caballero-Mellado J., Aguirre J.F., Kapulnik Y., Brener S., Burdman S., Kadouri D., Sarig S., Okon Y. Responses of Agronomically Important Crops to Inoculation with Azo-Spirillum. Functional Plant Biology, 2001; 28(9): 871–879.
CrossRef - El-Azeem, A. S., Mehana, T.A., Shabayek A.A.Effect of Seed Inoculation with Plant Growth-Promoting Rhizobacteria on the Growth and Yield of Wheat (Triticum aestivum L.) Cultivated in a Sandy Soil. Catrina: The International Journal of Environmental Sciences, 2008;3(2):69-74.
- Evangelista E.V., Garcia F.C., Cruz J.A. Isolation Characterization and Identification of Plant Growth-Promoting Rhizobacteria. Int J Agric Technol, 2017; 13(5): 715-727.
- Fahsi N., Mahdi I., Mesfioui A., Biskri L., Allaoui A. Plant Growth Promoting Rhizobacteria Isolated from the Jujube (Ziziphus Lotus) Plant Enhance Wheat Growth, Zn Uptake, and Heavy Metal Tolerance. Agriculture, 2021; 11(4): 316.
CrossRef - Fiodor, Ajijah N., Dziewit L.,Pranaw K. Biopriming of Seed with Plant Growth-Promoting Bacteria for Improved Germination and Seedling Growth. Front Microbiol, 2023; 14:1142966.
CrossRef - Ghavam S., Vahdati M., Wilson IAG., Styring P. Sustainable Ammonia Production Processes. Front. Energy Res, 2021; 9:34.
CrossRef - Hassan A. H., Mietzel T., Brunstermann R., Schmuck S., Schoth J., Küppers M., Widmann R. Fermentative Hydrogen Production from Low-Value Substrates. World Journal of Microbiology and Biotechnology, 2018; 34: 1-11.
CrossRef - Iyer B., Rajput M. S.,Rajkumar S. Effect of Succinate on Phosphate Solubilization in Nitrogen Fixing Bacteria Harbouring Chick Pea and their Effect on Plant Growth. Microbiol Res, 2017; 202: 43–50.
CrossRef - Kannahi M., Megala R. Phosphate Solubilizing Potentiality of Bacillus subtilis and Pseudomonas Aeruginosa on Vigna Unguiculata Growth using Agrowaste as a Substrate. World Journal of Pharmacy and Pharmaceutical Sciences, 2015; 4(8): 1238-1244.
- Kesaulya H., Talahaturuson A., Kalay A.M., Matatula E., Lawalatta I.J., Hehanussa M.L., Nendissa S.J.Characterization of Plant Growth Promoting Rhizobacteria of Maize. IOP Conf. Series: Earth and Environmental Science, 2021; 883(1): 012028.
CrossRef - Kloepper J.W., Lifshitz R., Zablotowicz R. M. Free-Living Bacterial Inocula for Enhancing Crop Productivity. Trends In Biotechnology, 1989; 7(2): 39-44.
CrossRef - Kumar S., Stecher G.,Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Molecular Biology and Evolution, 2016; 33(7):1870-1874.
CrossRef - Lihan S., Benet F., Husaini A. A. S. A., Apun K., Roslan H. A., Hassan H. Isolation and Identification of Plant Growth Promoting Rhizobacteria from Sago Palm (Metroxylon Sagu, Rottb.). Tropical Life Sciences Research, 2021; 32(3): 39-51.
CrossRef - Ludvigsen, J., Porcellato, D., Amdam, G. V., Rudi K. Addressing the Diversity of the Honeybee Gut Symbiont Gilliamella: Description of Gilliamella Apis Sp. nov., Isolated from the Gut of Honeybees (Apis Mellifera). International Journal of Systematic and Evolutionary Microbiology, 2018; 68(5):1762-1770.
CrossRef - Makhaye G., Aremu A. O., Gerrano A. S., Tesfay S., Du Plooy C. P., Amoo S. O. Biopriming with Seaweed Extract and Microbial-Based Commercial Biostimulants Influences Seed Germination of Five Abelmoschus Esculentus Plan. Theory, 2021; 10(7):1327.
CrossRef - Mayak, S., Tirosh, T.,Glick B. R. Effect of Wild-Type and Mutant Plant Growth-Promoting Rhizobacteria on the Rooting of Mung Bean Cuttings. Journal of Plant Growth Regulation, 1999; 18:49-53.
CrossRef - Naz A., Razzaq k., Raza N., Hussain M., Mujtaba A., Afzal M. Evaluation of Enzymatic and Non-enzymatic Antioxidant Potential of Sprouted Indigenous Legumes from Pakistan. International Journal of Food Properties, 2023; 26(1):1230-1243.
CrossRef - Nezarat S., Gholami A. Screening Plant Growth Promoting Rhizobacteria Improving Seed Germination, Seedling Growth and Yield of Maize. Pakistan Journal of Biological Sciences, 2009; 12(1): 26-32.
CrossRef - Patel M., Vurukonda S. S. K. P.,Patel A. Multi-Trait Halotolerant Plant Growth-Promoting Bacteria Mitigate Induced Salt Stress and Enhance Growth of Amaranthus Viridis. Journal of Soil Science and Plant Nutrition, 2023; 1-24.
CrossRef - Raj S. N., ShettyN. P., Shetty H. S. Seed Bio-Priming with Pseudomonas Fluorescens Isolates Enhances Growth of Pearl Millet Plants and Induces Resistance against Downy Mildew. International Journal of Pest Management, 2004; 50(1): 41-48.
CrossRef - Rana A., Saharan B., Joshi M., Prasanna R., Kumar K., Nain L. Identification of Multi-Trait PGPR Isolates and Evaluating their Potential as Inoculants for Wheat. Annals of Microbiology, 2011; 61(4): 893-900.
CrossRef - Reetha A. K., Pavani S. L., Mohan S. Hydrogen Cyanide Production Ability by Bacterial Antagonist and their Antibiotics Inhibition Potential on Macrophomina Phaseolina (Tassi.) Goid. J. Curr. Microbiol. Appl. Sci, 2014; 3(5): 172-178.
- Saitou N.,Nei M. The Neighbor-Joining Method: A New Method for Reconstructing Phylogenetic Trees. Molecular Biology and Evolution, 1987; 4(4): 406-425.
- Sarkar D., Rakshit A., Al-Turki A.I., Sayyed R.Z., Datta R. Connecting Bio-Priming Approach with Integrated Nutrient Management for Improved Nutrient Use Efficiency in Crop Species. Agriculture, 2021;11(4): 372.
CrossRef - Sharma A., Dev K., Sourirajan A., Choudhary M. Isolation and Characterization of Salt-Tolerant Bacteria with Plant Growth-Promoting Activities from Saline Agricultural Fields of Haryana, India. Journal of Genetic Engineering and Biotechnology, 2021; 19(1):1-10.
CrossRef - Shaukat K., Affrasayab S., Hasnain,S. Growth Responses of Triticum Aestivum to Plant Growth-Promoting Rhizobacteria Used as Biofertilizer. Res J Microbiol., 2010;5(10):1022-1030.
- Singh J.,Singh S.P. Isolation and Identification of Bacillus Species from Soil for Phosphate, Potassium Solubilization and Amylase Production. Int J Curr Microbiol App Sci, 2020; 9(5): 415-426.
CrossRef - Vaughn R. H., Osborne J. T., Wedding G. T., Tabachnick J., Beisel C. G., Braxton T. The Utilization of Citrate by Escherichia coli. Journal of Bacteriology,1950;60(2): 119-127.
CrossRef - Villacieros M., Power B., Sanchez-Contreras M., Lloret J., Oruezabal R. I., Martín M.,Rivilla R. Colonization Behaviour of Pseudomonas Fluorescens and Sinorhizobium Meliloti in the Alfalfa (Medicago Sativa) Rhizosphere. Plant and Soil, 2003;251: 47-54.
CrossRef - Zhang F., Dashti N., Hynes R. K., Smith D. L. Plant Growth-Promoting Rhizobacteria and Soybean [Glycine Max (L.) Merr.] Growth and Physiology at Suboptimal Root Zone Temperatures. Annals of Botany, 1997; 79(3): 243-249.
CrossRef

