Introduction
One of the fast-growing research areas in the heterocyclic chemistry has been directed towards the synthesis of isoxazoline derivatives; as a structural unit frequently found in natural products1 and many bioactive 2,3 molecules. For instance, cycloserine4 is a naturally occurring antituberculotic and antibiotic agent possessing isoxazoline nuclei. In the chemistry of five membered heterocycles, isoxazoline and their analogues have achieved great importance as being enabled to exhibit various biological activities viz. antibacterial,5 analgesic, anthelmintic,6 diuretic, anticonvulsant,7 anticancer, antitubercular,8-9 insecticidal, cardiovascular,10 antidepressant and anti-anxiety11 activities. Besides these, they are used as colorant and fastness agent for synthetic fabric,12 also showed herbicidal13 actively and growth promoting impact on some flowering plants.14-15
The greater efficacy of biological properties exhibited by isoxazoline moiety stimulated the research work to develop new synthetic approaches for their synthesis and study their activities. In addition to above mentioned biological activities, these can act as valuable intermediates for the synthesis of organic molecule. Variety of compounds possessing important functional groups such as β-hydroxynitrile, α, β-unsaturated carbonyl compound, β-hydroxynitrile, 1,3-diamines and 1,3-hydroxycarbonyl compounds were useful for the preparation of these compounds. Jadhav S.B. (2010)16 reported cyclization of substituted chalcones with hydroxylamine hydrochloride using sodium acetate in DMF which results into the formation of newly substituted 3-(2’-hydroxyphenyl)-5-(6’’-methoxy-naphthalen)-2-isoxazolines.
An important route for the synthesis of isoxazolines is 1,3-dipolar cycloaddition reaction, wherein nitrile oxides react with alkenes to yield isoxazolines. Alternative alkyl or silyl nitronates17 also used to achieve the same products. Thus, the reaction of alkyl or silyl nitronate with alkene affords N-alkoxy or N-silyloxyisoxazolidines. Further on heating or on acidic condition it gives isoxazolines.
Base catalysed cyclization of 2-hydroxyacetonaphthanone with substituted vanillin provides chalcone. Further this on treatment with NH2OH.HCl in basic medium using ethanol leading to the formation of corresponding isoxazoline analogues have been reported by Sailu et al. (2012),18 Similar types of reactions have also been reported by Solankee et al. (2015)19 with the use of s-triazine based chalcones and NH2OH.HCl in the presence of alkali. Literature survey also reveals that chromanones and flavanones are also useful starting materials in the synthesis of isoxazolines. Dhirbassi et al. (2014)20 carried out reactions of bromo-nitro-substituted flavanones with hydroxylamine -hydrochloride in pyridine containing few drops of piperidine to synthesise 3,5-diaryl-4-aroyl isoxazolines.
From the review of literature, it is revealed that the synthesis of imidazole substituted isoxazolines from 2-aminosubstituted chloroflavonones proved to be useful pathway. Thus, it was thought interesting to carry out the reaction of 2-aminosubstituted-chloroflavonone with NH2OH.HCl in 1,4-dioxane/piperidine solvent mixture and study their impact on phytotic growth of mushroom spp. and also against some pathogens.
Materials and Methods
The materials prepared were characterised using various characteristics techniques. The UV-Vis spectra taken in ethanol solvent. Perkin-Elmer spectrophotometer and Bruker Avance-II 400 NMR spectrophotometer were used for IR spectra and 1H NMR spectra in CDCl3 using TMS as an internal standard respectively.
Preparation of 3-(substitutedphenyl)- 4-benzoyl-5-N[(substitutedphenyl)-ethanonyl amino]-Δ2-isoxazoline (3):
When mixture of 3-(2,5-disubstitutedphenyl)-4-benzoyl-5-amino-Δ2-isoxazoline (1) and substituted-2-bromoethanone (2) was boiled for 1 hour in absolute ethanol. On decomposition using ice-cold water yields product. Which was filter and crystallise in EtOH to obtain (3).
Mol. For. C24H18N2O5Cl2: Shining yellow amorphous solid, m. p. 164 oC, yield 69 %, Elemental analysis (%): C 59.34/59.40; H 3.70/3.74; N 5.75/5.77; O 16.39/16.48; Cl 14.58/14.61. UV (ethanol) λmax n→ π* = 390 nm; IR (KBr) (cm-1): 3590-2900 (-OH stret H-bonded.); 3079.62 (Aromatic -H), 2915.65 (C-H), 1619.10 (C=O), 1601.39 (C=N), 772.38 (C-Cl), 683.45 (C-Cl); 1HNMR (δ ppm): 1.62 (s,2H, -CH2), 1.2 (v, 1H,N-H), 6.8 (s, 1H, CH=C-OH), 7.93 (-CH-CH-CO-Ph), 7.95 (-CH-CH-CO-Ph), 7.2-8.1 (m, 11H, Aromatic -H).
Preparation of 3-(substitutedphenyl)-4-benzoyl-5-[2-mercapto-4-(substitutedphenyl) imidazolo]-Δ2-isoxazoline (4):
When mixture of 3-(substitutedphenyl)- 4-benzoyl-5-N[(substitutedphenyl)-ethanonyl amino]-Δ2-isoxazoline (3) and KSCN was boiled for 4 -4.15 hours using glacial acetic acid solvent. On decomposition using ice-cold water the product get separated and crystalised using EtOH to obtained titled compound (4).
Mol. For. C25H17N3O4SCl2: Yellow solid, mp 178 oC, yield 63 %, Elemental analysis (%):C 56.97/57.04; H 3.22/3.26; N 7.93/7.98; O 12.13/12.16; S 6.04/6.09; Cl 13.43/13.47. UV (ethanol) λmax n→ π* = 380 nm; IR (KBr) (cm-1): 3600-2600 (H-bonded -OH), 3085.18 (C-H), 2917.19 (C-H), 1650.50 (C=O), 1600.10 (C=N), 1131.15 (C-O), 771.10 (C-Cl), 681.12 (C-Cl). 1H NMR (δ ppm): 1.6 (-S-H), 6.8 (- CH-CH-CO-Ph), 7.2 (-CH-CH-CO-Ph), 7.5-8.2 (Aromatic -H).
Preparation of 3-(2-acetyloxy-substitutedphenyl)-4-benzoyl-5-[2-mercapto-4-(2’-acetyloxy-substitutedphenyl) imidazolo]-Δ2-isoxazoline (5):
When 3-(substitutedphenyl)-4-benzoyl-5-[2-mercapto-4-(substitutedphenyl) imidazolo]-Δ2-isoxazoline (4) and acetic anhydride boiled in acetic acid solvent for 45-50 min. On decomposition yield product. Which was crystallized using EtOH to obtain titled compound (5).
Mol. For. C29H21N3O6SCl2: White amorphous solid, m.p.138oC, yield 74 %, Elemental analysis (%): C 56.97/ 57.06; H 3.42/ 3.47; N 6.85/ 6.88; O 15.69/ 15.73; S 5.23/ 5.25; Cl 11.57/ 11.62.
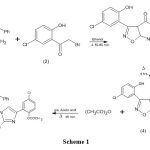 |
Scheme 1 |
Antimicrobial Activity
The compounds prepared were tested against mushroom crop pathogens. The result obtained from this study are tabulated in Table 1. Tested compounds when compared with reference compound had been shown good to moderate efficacy against species under studied. viz. Verticillium fungicola, Gliocladium roseum Burkholderia gladioli. Pseudomonas fluorescene, Pseudomonas alcaligens and Pseudomonas stutzeri.
Table 1: Efficacy of titled compounds against Oyster mushroom crop pathogens.
| S.N. | Compounds | Zone of inhibition in mm | |||||
| Fungi | Bacteria | ||||||
| V. fungicola | G. roseum | B. gladioli | P. fluorescene | P. alcaligens | P. stutzeri | ||
| 1. | 1 | 07 | 07 | 10 | 08 | 08 | 08 |
| 2. | 2 | 07 | 07 | 06 | 07 | 09 | 06 |
| 3. | 3 | 10 | 15 | 14 | 12 | 14 | 09 |
| 4. | 4 | 10 | 05 | 06 | 09 | 13 | 09 |
| 5. | Carbendizium | 09 | 09 | NA | NA | NA | NA |
| 6. | Gentamycine | NA | NA | 08 | 08 | 08 | 08 |
Efficacy of Test Compounds on Growth of Mushroom Spp.
Experimental setup was established at ICAR affiliated Krushi Vidyan Kendra, Durgapur (Badnera) Dist. Amravati under the consultancy of eminent experts. The mushroom spp. Pleurotus sajor-caju was selected for the study. The spawns of experimental species were procured from genuine agricultural agencies and cultivated in the culture house. The experimental setup was divided into two parts ie ‘A’-control group plants and ‘B’-treated group plants.
Firstly, the substrate soybean straw was chopped into small pieces and keep for 10-14 hours in water tank for soaking purpose. Further soaked substrate was sterilized by treating it with 60-80 oC hot water for an hour. After sterilisation, substrate was cool to lower down the temperature.
Polythene bags (sterilized) were used to prepared uniform size beds. Sterilized soybean straw and spawns treated with the test compounds solution were filled one by one in bags. The beds (packets) closed tightly with threads and made pin-holes around the bag. Likewise, a bag for controlled group (untreated spawns) was filled and labelled. All the packets under study were hanged in cultivation room for incubation at 22-26 oC for mycelium running for 21-28 days. Care has been taken to maintain proper temperature of the incubation room. Within 25-30 days mycelium developed around the bag, polyethene was removed and transfer to growing room, where bets were irrigated as per their need.
As soon as, the first primordial initiated, the solution of test compounds were sprayed on it and continued with some intervals. The yields of mushroom crop were collected from each bag was studied with reference to their diameter, length, weight and colour and recorded as shown in table 2:
Table 2: Effect of titled compounds on Oyster mushroom: Pleurotus sajor-caju spp.
| Sl.No. | Compo-
unds |
D (cm) | T (cm) | L (cm) | Weight of
Dry Bags (gm) (After Harvesting) |
Total Weight (gm) | Colour | |
| Fresh | Dry | |||||||
| 1. | 1 | 7.0 | 0.6 | 5.7 | 0.932 | 214 | 21.41 | White |
| 2. | 2 | 8.8 | 0.4 | 5.8 | 0.987 | 196 | 17.23 | White |
| 3. | 3 | 11.5 | 0.7 | 6.8 | 0.989 | 224 | 20.30 | Creamy |
| 4. | 4 | 11.4 | 0.5 | 6.2 | 0.962 | 202 | 19.00 | Creamy |
| 5. | 1,4-Dioxane | 6.0 | 0.4 | 6.1 | 0.991 | 174 | 19.12 | White |
| 6. | Control | 6.8 | 0.3 | 5.5 | 0.853 | 204 | 20.00 | White |
| D = Diameter; T = Thickness; L = Length | ||||||||
Results and Discussion
In this study newly synthesized 3-(2,5-disubstitutedphenyl)-4-benzoyl-5-amino-Δ2-isoxazoline (1), substituted-2-bromoethanone (2), 3-(substitutedphenyl)- 4-benzoyl-5-N[(substitutedphenyl)-ethanonyl amino]-Δ2-isoxazoline (3), 3-(substitutedphenyl)-4-benzoyl-5-[2-mercapto-4-(substitutedphenyl) imidazolo]-Δ2-isoxazoline (4) were tested for their efficacy towards antimicrobial activity specialy against pathogens causing damages to Mushroom crop mentioned in table no. 2. From the study, it is concluded that, the heterocycles chosen for the study showed very good to moderate amount of antibacterial activity.
Pleurotus sajor-caju was treated with the solution of test compounds to examine their efficacy on the morphology of treated mushroom species. By considering the morphological characters, comparison have been made between the treated and control species of mushroom, it was noticed that, the mushroom species which was under treatment showed notable growth in caps diameter as well as thickness also increased in stipe length that contributes the enhanced crop production.
Acknowledgement
The authors acknowledge NCIM, NCL, Pune and MTCC, CSIR, Chandigarh for cooperation received for the supply of microbial culture, Department of Microbiology, S.K. College, Akola for providing laboratory facilities. We acknowledge CIL and SAIF, Panjab University, Chandigarh for providing the spectral data. We also acknowledge Dr.K.A.Dhapke and Dr.A.N.Kakade, KVK, Durgapur (Badnera) Amravati for their guidance and consultancy in the study of growth impact on mushroom as well as in the analysis of study samples.
References
- Goncalves R.S.B., Santos M.D., A one-pot synthesis of 3-trifluoromethyl-2-isoxazolines from trifluoromethyl aldoxime, Beilstein J Org Chem, 2013; 9, 2387-2394.
CrossRef - El Sayed, K. A.; Bartyzel, P.; Hamann, M. T., Marine natural products as antituberculosis agents, Tetrahedron, 2000; 56 (7): 949–953.
CrossRef - Proksch, P.; Putz, A.; Ortlepp, S.; Bioactive natural products from marine sponges and fungal endophytes, Bayer, M. Rev. 2010; 9: 475–489.
CrossRef - Gillchrist T.L., Heterocyclic Chemistry, 3rd, Pearson publication, New Delhi. ISBN: 978-81-317-0793-7.
- Al-Wabli R. I., Al-Ghamdi A. R., Ghabbour H. A, Al-Agamy M. H, Attia M. I., Synthesis and spectroscopic identification of certain imidazole-semicarbazole conjugates bearing benzodioxole moieties: New antifungal agent, Molecule, 2019; 24(1): 200.
CrossRef - Hong-liang Yang, Bo Jin, Jia-yi Chen, Zun-lai Sheng and Meng-qing Sun, Synthesis, antibacterial and anthelmintic activity of novel 3-(3-pyridyl)-oxazolidinone-5-methyl ester derivatives, Molecules, 2022; 27: 1103.
CrossRef - Minu M., Singh D. QSAR modeling on quinazolinonyl pyrazolines and quinazolinoyl isoxazolines as anticonvulsant agents, Adv. Pharm. Edu. & Res., 2014; 4(1): 59-65.
- Harinadha B.V., G Priyanka, Naresh K, Ulayain N. and Reddy B.M., Synthesis and antitubercular activity of isoxazole incorporated 1,2,3-triazole derivatives, RJPBCS, 2016; 7(2): 1167-1171.
- Tangallapally R.P., Sun D., Lenaerts J., Meibohm B., Lee R.E., Discovery of novel isoxazolines as anti-tuberculosis agents, Bioorg Med Chem Lett, 2007; 17(23): 6638-6642.
CrossRef - Anila Kumari V S, Prasobh G R, Sheeja Rekha A G, Athira A S, Seba M C, Gini Jameena Y, A brief review on isoxazole derivatives as antibacterial agents, International Journal of Research and Review, 2022; 9(9): 321-333.
CrossRef - Kumar J., Chawla G., Tanwar O., Bhowmik M., Synthesis and neuropharmacological evaluation of some new isoxazoline derivatives as antidepressant and anti-anxiety agents, Afr. J. Pharm. Pharmacol., 2013; 7(22): 1523-1530.
CrossRef - Kiran V. Mehta, Studies on some isoxazoline-azo compounds and their colourant performance and fastness evaluation on synthetic fabric, J. Chem. Tech Res., 2012; 4(1): 409-414.
- Lee J.N., Koo S.J., Hwang K.H., Hwang I.T., Jeon D.J., Kim H.R., Proceedings of the 21st Asian Pacific Weed Science Society Conference, Colombo, Sri Lanka, October 2-6, 591-601, 2007.
- Boob S.D., Rajput P.R., Oriental Journal of Chemistry, A facile solvent free microwave induced synthesis of chlorine containing pyrazoline and Isoxazoline derivatives and their phytotic impact on some flowering plants and antimicrobial activity, 2010; 26(3): 879-889.
- Parihar R.T., Bhoyar A.D., Rajput P.R., A novel functions for 4-aroylpyrazolines and isoxazolines in growth promoting effects on some horticultural crops, IJCPS, 2012; 1(2): 54-59.
CrossRef - Jadhav S.B., Synthesis and antimicrobial studies of some novel isoxazoline derivatives IJABPT, 2010; 1(3): 939-945.
- Henry Feuer, Nitrile oxides, nitrones & nitronates in organic synthesis: novel strategies in synthesis. 2nd edition, John Wiley & Sons, Inc., Hoboken, New Jersey. 2007.
CrossRef - Sailu B., Mahanti S., Satya S.A, Balram B., Ram B., Taara B., Vasudha B., Synthesis and antibacterial activity of novel isoxazoline derivatives, Der Pharma Chemica, 2012; 4(5): 2036-2041.
- Solankee A., Tailor R., Synthesis, characterisation and biological screening of s-triazine based chalcones and its derivatization into phenyl pyrazolines, isoxazoles, International Letters of Chemistry, Physics and Astronomy, 2015; 47: 109-119.
CrossRef - Dhirbassi S.D., Dighade S.R., Synthesis of substituted bromo-nitroflavanones and 3, 5-diaryl-4-aroyl isoxazolines, Rasayan J. Chem., 2014; 7(1): 33-38.

